Pyrimidine-2,4-diamine derivative and anticancer pharmaceutical composition comprising same as effective ingredient
一种药学、化合物的技术,应用在癌症的预防或治疗用药学组合物领域,达到防止癌症复发、优秀抑制间变性淋巴瘤激酶活性的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
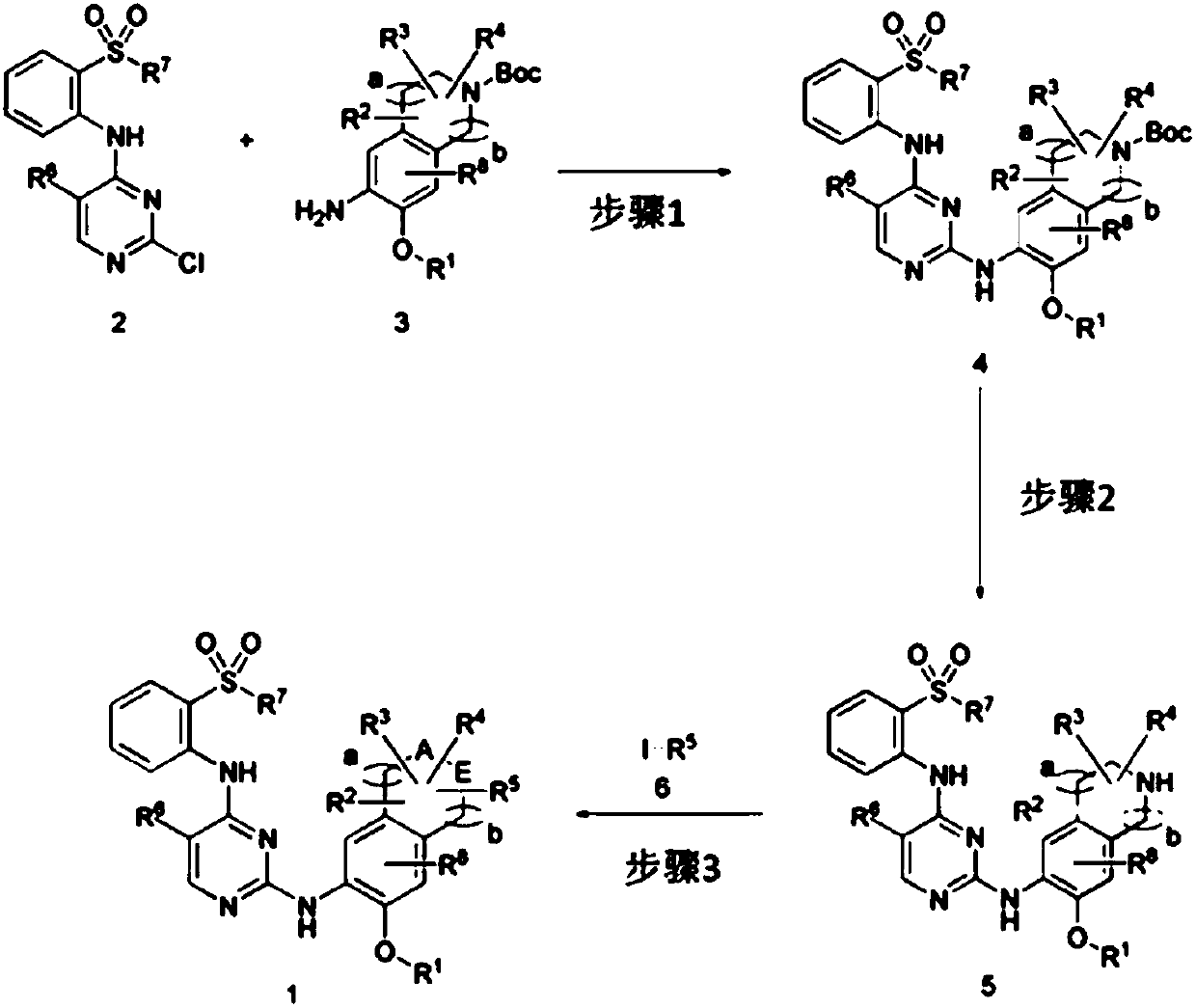
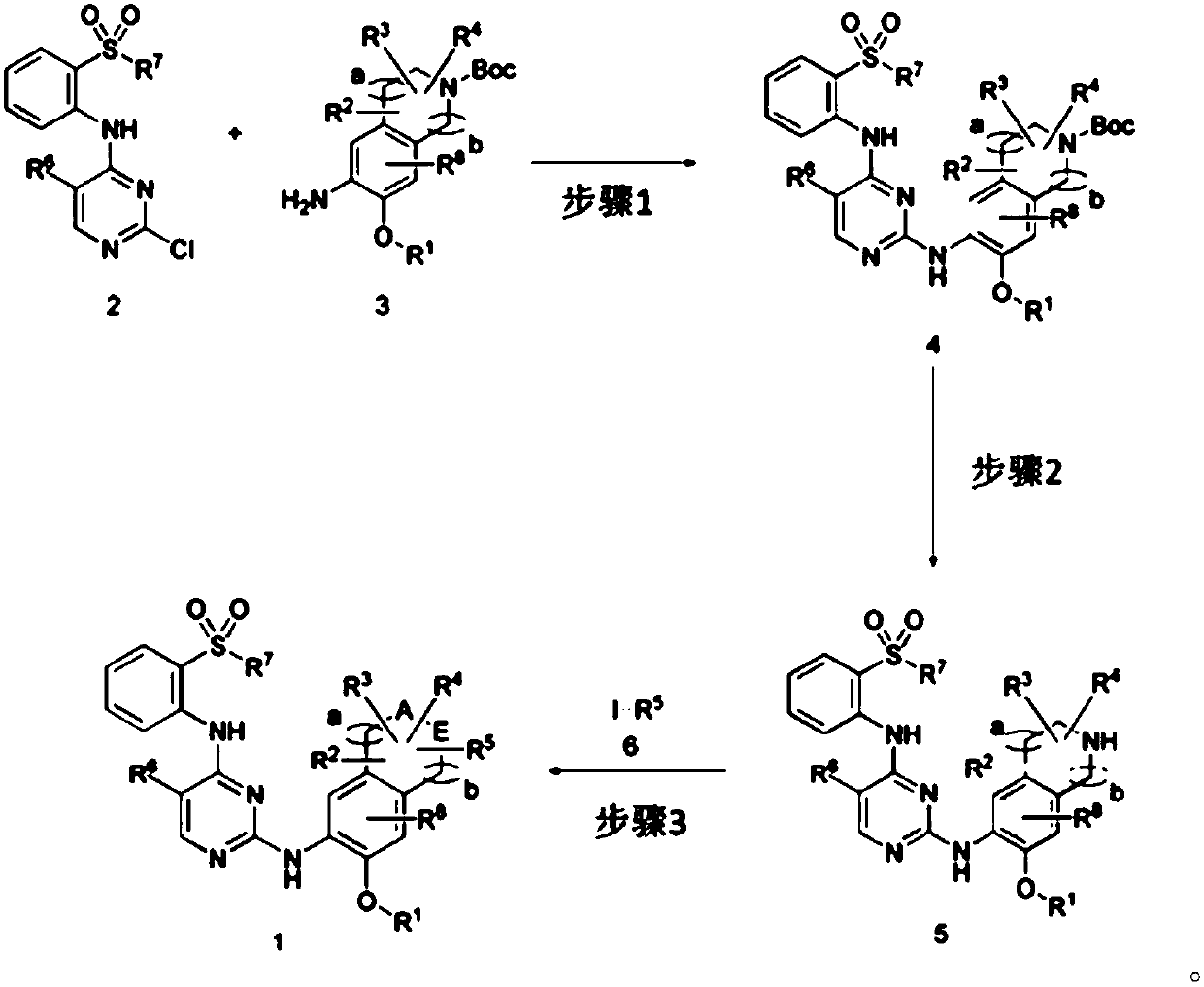
[0182]And, the present invention provides the preparation method of the compound represented by above-mentioned chemical formula 1, as shown in following reaction formula 1, the preparation method of above-mentioned compound comprises: Step 1, make the compound represented by chemical formula 2 and the compound represented by chemical formula 3 react to prepare the compound represented by Chemical Formula 4; Step 2, to prepare the compound represented by Chemical Formula 5 by replacing the -Boc group of the compound represented by Chemical Formula 4 obtained in the above Step 1 with hydrogen; and Step 3, making the compound represented by the Chemical Formula 5 in the above Step 2 The compound represented by Chemical Formula 5 obtained in and the compound represented by Chemical Formula 6 were reacted to obtain the compound represented by Chemical Formula 1.
[0183] Reaction 1:
[0184]
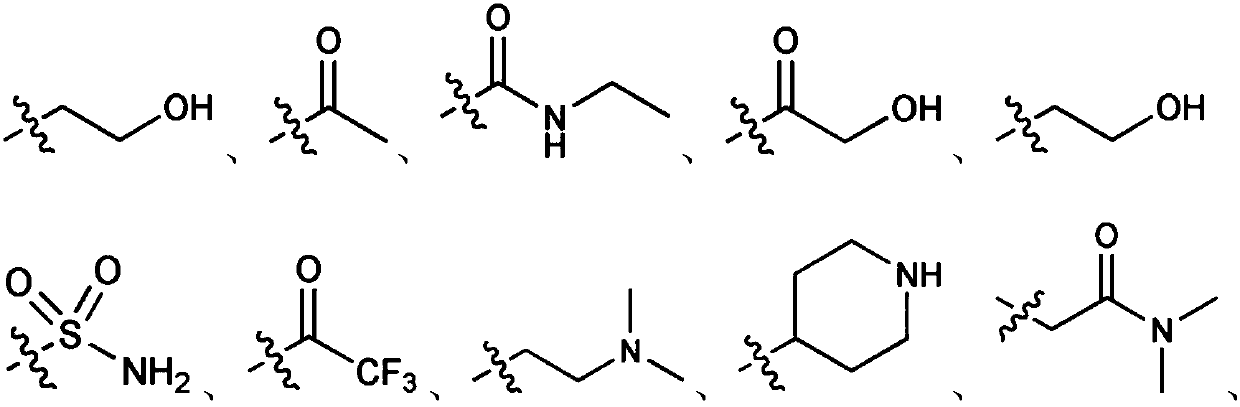
[0185] In the above reaction formula 1, -Boc is or R 1 , R 2 , R 3 , R 4 , R ...
preparation example 1
[0274] Preparation of tert-butyl-6-amino-7-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate
[0275] Step 1: Preparation of 2,2,2-trifluoro-N-(4-methoxyphenylethyl)acetamide
[0276]
[0277] After dissolving 4-methoxyphenylethylamine (12.0 g, 79.4 mmol) in dichloromethane, trifluoroacetic acid anhydrate (13.5 mL, 95.2 mmol) was added.
[0278] The temperature was lowered to 0°C, triethylamine (27.6 mL, 198 mmol) was slowly added, and stirred at room temperature for 3 hours. After completion of the reaction, water was added for dilution, extracted twice with ethyl acetate, dried over anhydrous magnesium sulfate, and then concentrated under pressure. The filtrate which was concentrated under pressure was purified by column chromatography, and the target compound (19 g, 97%) was obtained.
[0279] 1H-NMR (300MHz, DMSO-d 6 )δ2.72(t, J=7.2Hz, 2H), 3.35-3.39(m, 2H), 3.71(s, 3H), 6.87(d, J=8.4Hz, 2H), 7.11(d, J=8.7 Hz, 2H), 9.46(s, 1H);
[0280] LC / MS 248.30[M + +H].
[...
preparation example 2
[0306] Preparation of tert-butyl-7-amino-6-methoxy-3,4-dihydroisoquinoline-2(1H)-carboxylate
[0307] Step 1: Preparation of methyl 3-methoxyphenylethyl carbamate
[0308]
[0309] After dissolving 3-methoxyphenylethylamine (30.0 g, 198 mmol) in dichloromethane, sodium carbonate (42.1 g, 396 mmol) was added. Methyl chloroformate (18.6 mL, 238 mmol) was added slowly at a temperature of 0°C and stirred for 2 hours. After completing the reaction, the organic layer was separated by adding water, and dried over anhydrous magnesium sulfate. Concentration under pressure was performed to obtain the target compound (35 g, 86%).
[0310] 1H NMR (300MHz, CDCl 3 )δ2.76(t, J=6.9Hz, 2H), 3.41(t, J=6.9Hz, 2H), 3.66(s, 3H), 3.80(s, 3H), 3.92(s, 3H), 4.69( br s, 1H), 6.74-6.79(m, 3H), 7.20-7.26(m, 1H);
[0311] EI / MS 209.1 [M+].
[0312] Step 2: Preparation of 6-methoxy-3,4-dihydroisoquinolin-1(2H)-one
[0313]
[0314] Polyphosphoric acid (184mL, 184mmol) was put into the reacti...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap