Bibenzyl ether compound as well as preparation method and application thereof
A compound and solvate technology, applied in the field of bibenzyl ether compounds and their preparation, can solve the problems of no bibenzyl ether compound research reports and no bibenzyl ether compound antitumor drug research reports, etc. Achieve significant inhibition of activity, high yield, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
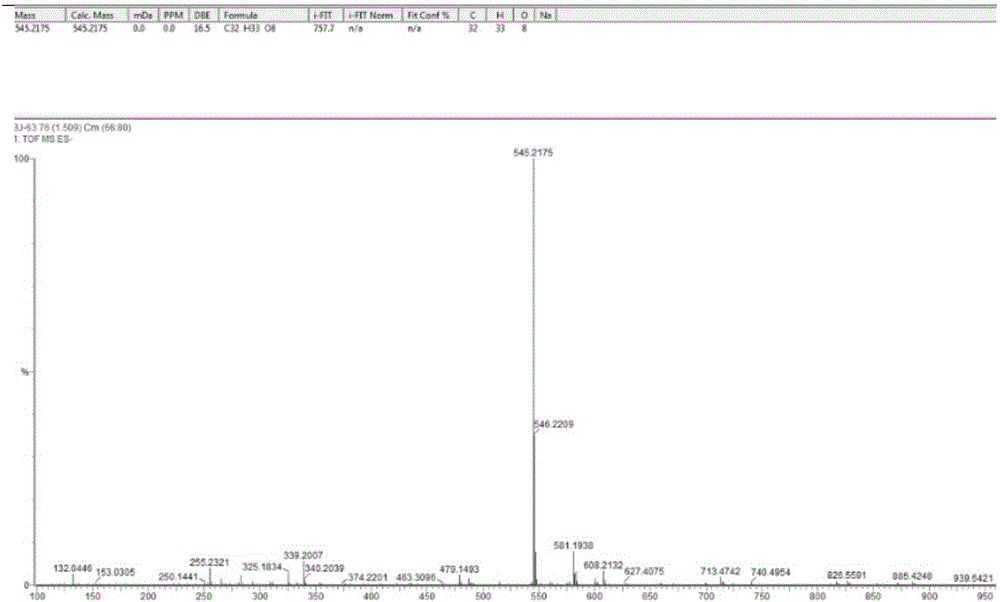
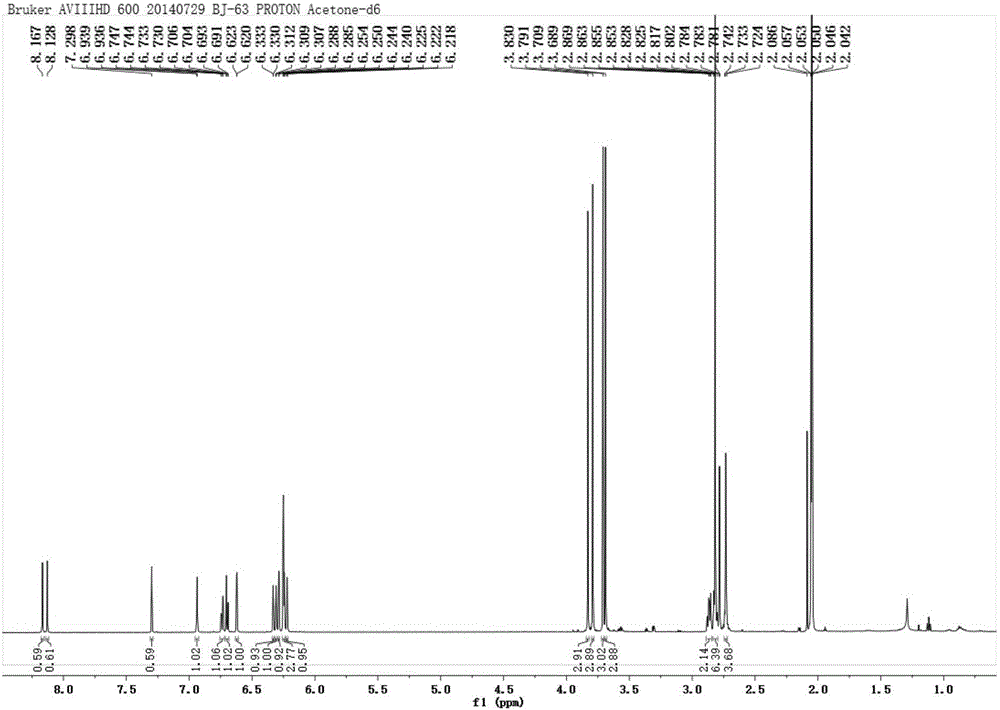
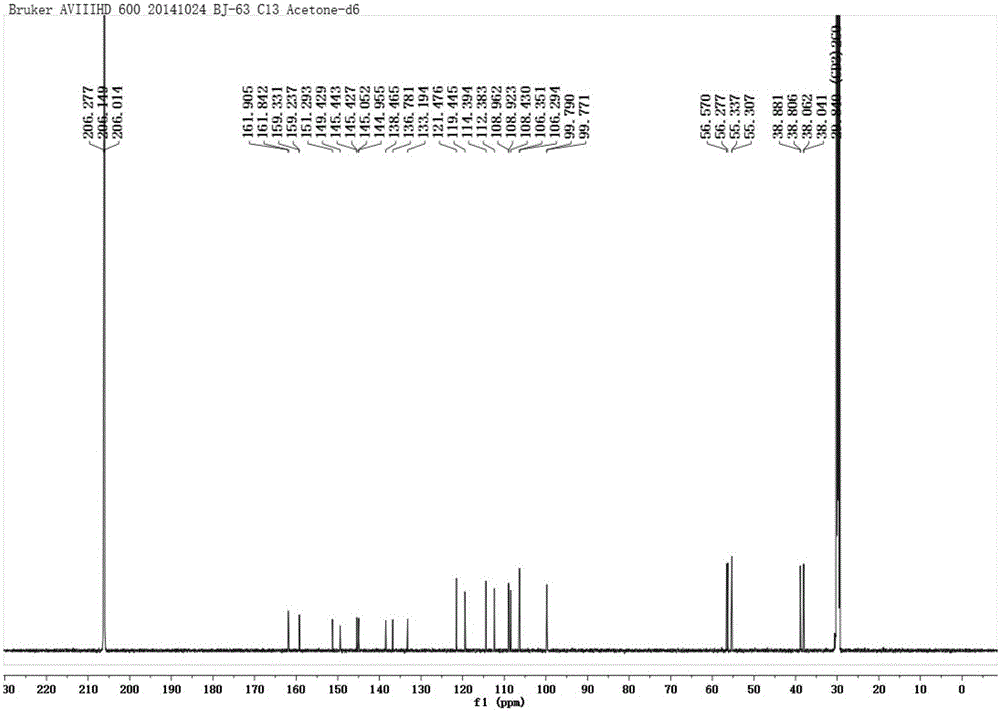
[0088] Example 1. Extraction and structural identification of bibenzyl ether compounds of the present invention
[0089] (1) Extraction of medicinal materials:
[0090] Dried white and medicinal material coarse powder (3kg), reflux extraction with 95% ethanol 3 times (30L×3), 2h each time;
[0091] (2) Separation and purification of components:
[0092] 1. Ethanol extract (90L) was concentrated and dried under reduced pressure to obtain 510g of semi-solid liquid extract;
[0093] ②. Disperse the semi-solid liquid extract (500g) with water (5L), extract with ethyl acetate (20L) and n-butanol (20L) successively, combine the ethyl acetate part, recover the solvent under reduced pressure, and obtain ethyl acetate leaching Cream 160g;
[0094] 3. Adopt silica gel column chromatography to separate ethyl acetate extract (160g), petroleum ether: acetone carries out gradient elution, and the eluent is detected by thin-layer chromatography, and obtains the elution when petroleum ethe...
Embodiment 2
[0112] Example 2, the anti-lung cancer A549 activity test of the bibenzyl ether compounds of the present invention
[0113] ① Cell inoculation
[0114] Cells in logarithmic growth phase were digested with 0.25% trypsin. Cultured in cell culture medium containing 10% FBS to form a single cell suspension. The A549 tumor cells in good condition were inoculated in a 96-well plate by counting with a cell counting plate, so that the cell density was 4×10 3 cells / mL, add 100 μL of cell suspension to each well, place at 37°C, 5% CO 2 Cultivate in the incubator for 24h.
[0115] ②, drug treatment
[0116] Starting from 10 μg / mL for each sample, dilute the sample with medium gradient, 2-fold dilution, set 5 drug concentrations, and do a duplicate hole test for each concentration. Add 100 μL of drug to each well at a concentration of 10, 5, 2.5, 1.25, 0.625 μg / mL, set 3 replicate wells for each concentration, and repeat 3 times. The negative control group is a medium solution conta...
Embodiment 3
[0121] Embodiment 3, anti-acute monoleukemia MV4-11 activity test of bibenzyl ether compounds of the present invention
[0122] ① Cell inoculation
[0123] Cells in logarithmic growth phase were digested with 0.25% trypsin. Cultured in cell culture medium containing 10% FBS to form a single cell suspension. The MV4-11 cells in good condition were inoculated in a 96-well plate by counting with a cell counting plate, so that the cell density was 2×10 4 cells / mL, add 100 μL of cell suspension to each well, place at 37°C, 5% CO 2 Cultivate in the incubator for 24h.
[0124] ②Drug treatment
[0125] Starting from 50 μg / mL for each sample, dilute the sample with culture medium step by step, 2-fold dilution, set 5 drug concentrations, and do a duplicate hole test for each concentration. Add 100 μL of drug to each well at the concentration of 50, 25, 12.5, 6.25, 3.125 μg / mL, set 3 replicate wells for each concentration, and repeat 3 times. The negative control group is a medium ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap