A method for detecting, screening and identifying metabolic biomarkers of kidney yang deficiency syndrome
A biomarker, kidney-yang deficiency technology, applied in biological tests, measuring devices, material testing products, etc., can solve the problem of not being able to determine kidney-yang deficiency syndrome biomarkers and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0063] Specific Embodiment 1: A method for detecting, screening and identifying metabolic biomarkers of kidney-yang deficiency syndrome according to this embodiment is specifically prepared according to the following steps:
[0064] Step 1: Replicate the rat model of kidney yang deficiency; subcutaneously inject olive oil solution into each clean-grade rat in the normal group, and subcutaneously inject 10 mg / mL cortex into each clean-grade rat in the model group at 0.1 mL / Kg body weight Ketone olive oil solution; collect urine samples, blood samples and organ samples of clean rats in normal group and model group;
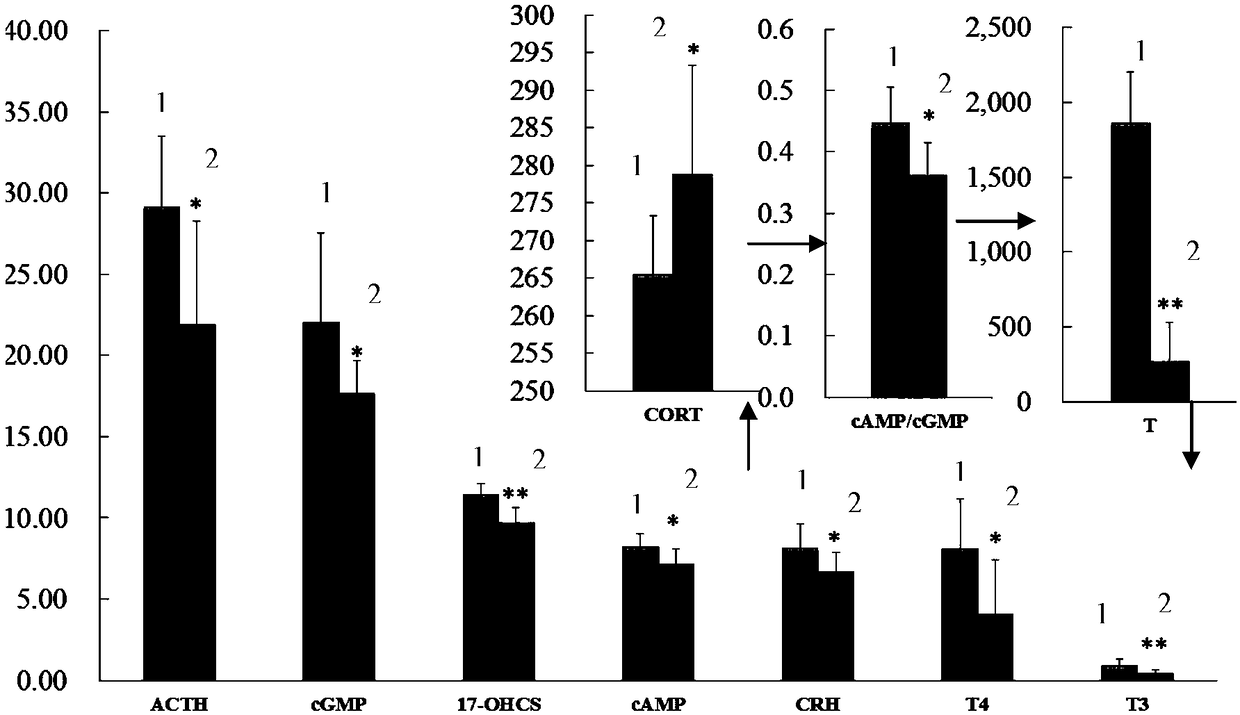
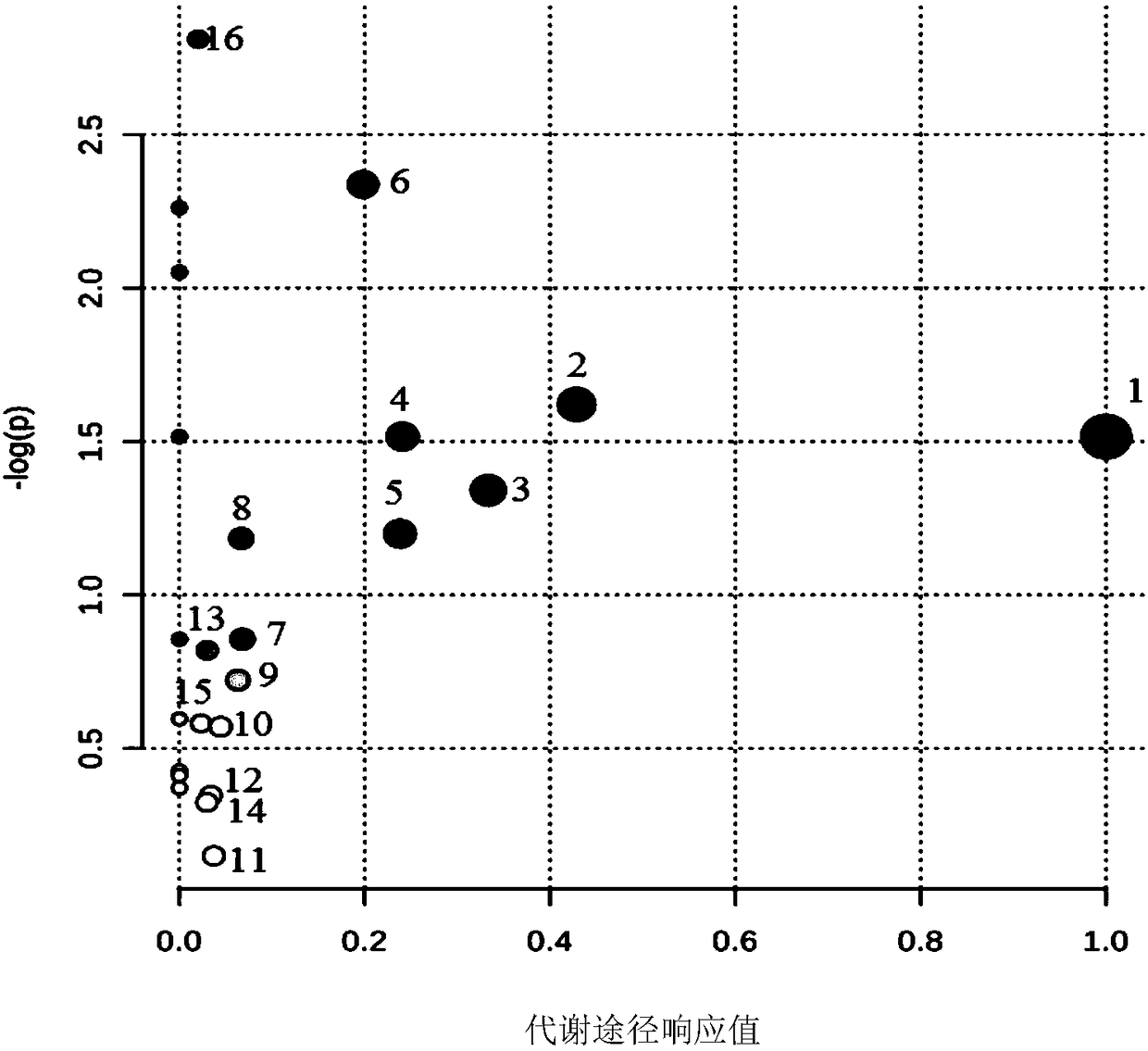
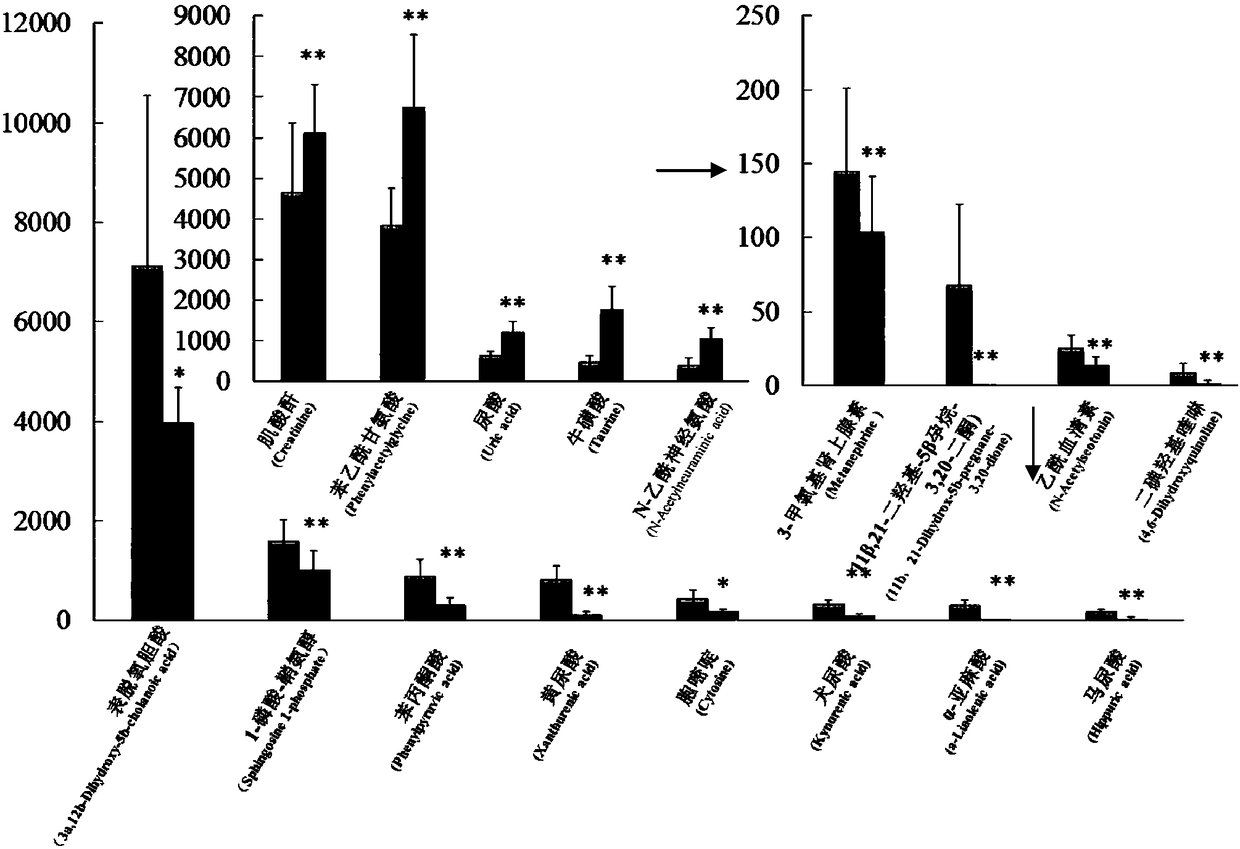
[0065] Step 2. Evaluate the rat model of kidney-yang deficiency; the clinical location of kidney-yang deficiency is the inhibition of the neuroendocrine immune network, and the corresponding hormone levels and the functional status of the target organs involved in the neuroendocrine immune network can reflect whether it is In a suppressed state; therefore, the clini...
specific Embodiment approach 2
[0082] Specific embodiment 2: The difference between this embodiment and specific embodiment 1 is that the urine samples collected from the normal group and the model group are specifically:
[0083] (1), the clean level rats are completely randomly divided into two groups, i.e. normal group and model group;
[0084] (2), each rat of normal group is injected olive oil solution subcutaneously by 0.1mL / Kg body weight, and each rat of model group is injected 10mg / mL corticosterone olive oil solution subcutaneously by 0.1mL / Kg body weight,
[0085] (3) Urine samples were collected from each rat for 12 hours, and the fresh urine samples were stored at -80°C under the conditions of centrifugation at 4°C and 13,000 rpm for 10 minutes, and thawed at room temperature before analysis. Other steps and parameters are the same as those in Embodiment 1.
specific Embodiment approach 3
[0086] Specific embodiment three: the difference between this embodiment and specific embodiment one or two is: the blood samples collected from the normal group and the model group of clean rats are specifically:
[0087] 1) Blood was collected from the abdominal aorta of each rat, and the serum was separated under the conditions of centrifugation at 4°C, 4000r / min, and 10min;
[0088] 2) Aliquoted and stored in a -80°C refrigerator, and thawed at room temperature before analysis. Other steps and parameters are the same as those in Embodiment 1 or Embodiment 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| collision energy | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com