Methods, devices and systems for pulmonary delivery of active agents
A pharmacological active agent and lung technology, applied in aerosol delivery, organic active ingredients, muscular system diseases, etc., can solve problems such as limitations, difficult quantification, and difficult controllable administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 4
[0675] Pulmonary Delivery Protocol
[0676] The following are examples of uses of the methods, apparatus and systems presented herein according to some embodiments of the present disclosure.
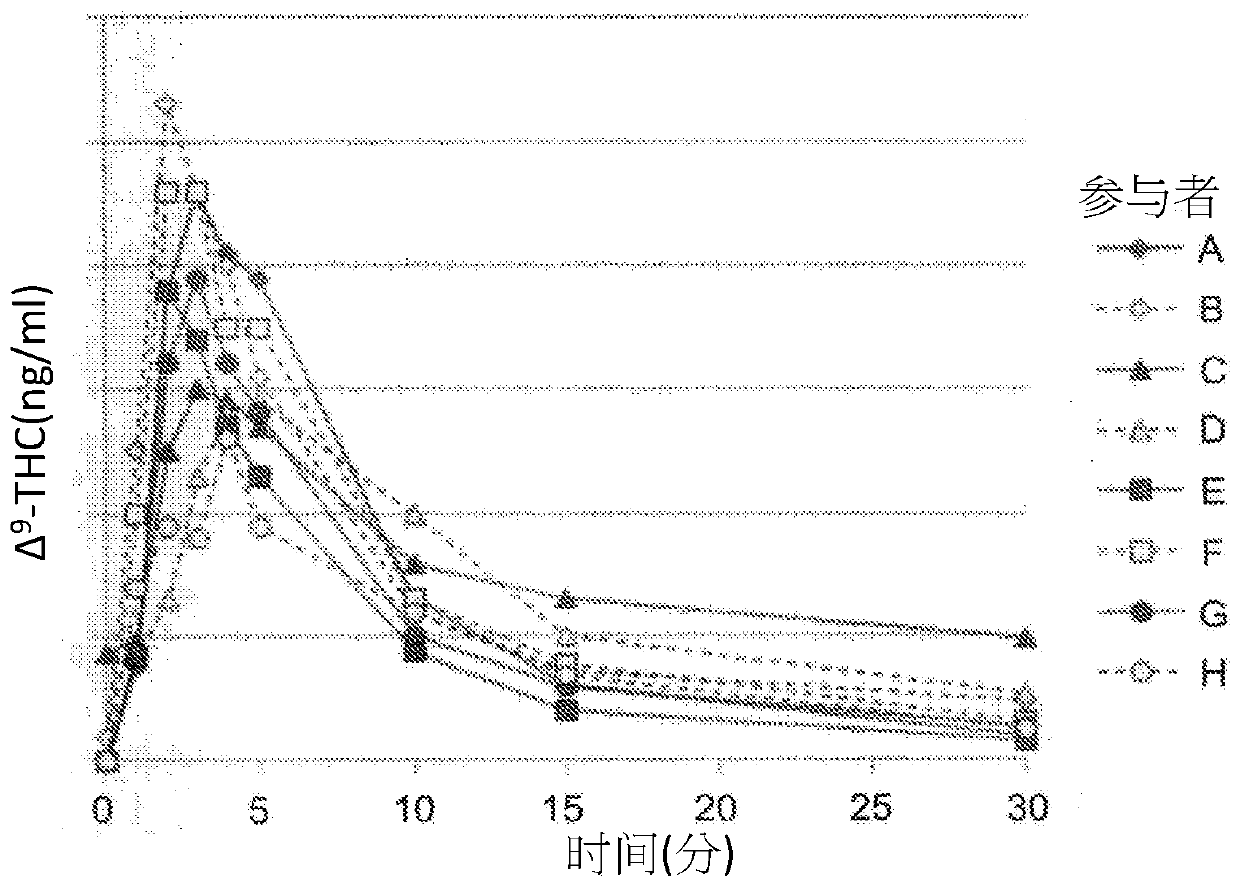
[0677] An MDI device, such as Figure 16 and / or as described in 17, loaded with a plurality of pre-weighted cartridges, each containing a substance comprising one or more volatilizable pharmaceutically active agents. As described above, the MDI is pre-calibrated and preset to deliver a predetermined vaporized amount of the pharmaceutically active agent based on PK / PD data obtained for a particular patient population and / or PK effects.
[0678] For example as above in Figure 9 This system, described in , is preloaded with personal information about the patient (e.g., PK variables) and his or her medical condition and history, data about the scheduled vaporized volume and time interval for pulmonary delivery of the agent (dosing and regimen); data on a preselected pharmacokinetic profi...
Embodiment 5
[0691] Treatment with combinations of active agents
[0692] According to some embodiments of the present disclosure, active agent combination therapy may include active agents that provide a synergistic effect; active agents that provide the same effect but each have different advantages or disadvantages; one active agent that enhances or weakens another or other Active (eg, altering each other's effective therapeutic window or therapeutic index); active agents that provide contradictory effects.
[0693] According to some embodiments, potential benefits of combination therapy, such as achieved through use of the presently described devices, include, but are not limited to, precise dosing of each of the multiple active agents as well as each inhalation event and between and / or inhalation of the active agents. Exact scaling between events.
[0694] For example, in some embodiments, the ability to deliver multiple regimens involving different agents and / or different dosing int...
Embodiment 6
[0711] User / physician interface input and output
[0712] The following is a presentation of user interface input categories according to some embodiments of the present disclosure.
[0713] Typically and optionally, the user interface may provide feedback from the user indicating one or more of the following:
[0714] 1. User identity;
[0715] 2. Perceived or measured treatment / dose / regime treatment or adverse effects;
[0716] 3. Instructions / requests from the user regarding timing and / or acceptable and / or expected treatments or adverse effects.
[0717] User identity may be monitored by user response (password, pattern entry or other challenge request) or by biometric means (facial and / or voice recognition, fingerprint, retinal scan). Usage activity (device hold / grip, inhalation, inactivity) can be monitored through thermal sensing, charge and acceleration measurements.
[0718] Perceived or measured treatment or side effects may include one or more of treatment relate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| electrical resistivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com