Method for preparing crizotinib intermediate by using carbonyl reductase
A crizotinib and reductase technology, which is applied in the field of preparing crizotinib intermediates by using carbonyl reductase, can solve the problems of cheap preparation of unfavorable intermediates, unsuitable large-scale production, low product yield and the like, and achieves important industrial Application value, the effect of small amount of biocatalyst and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
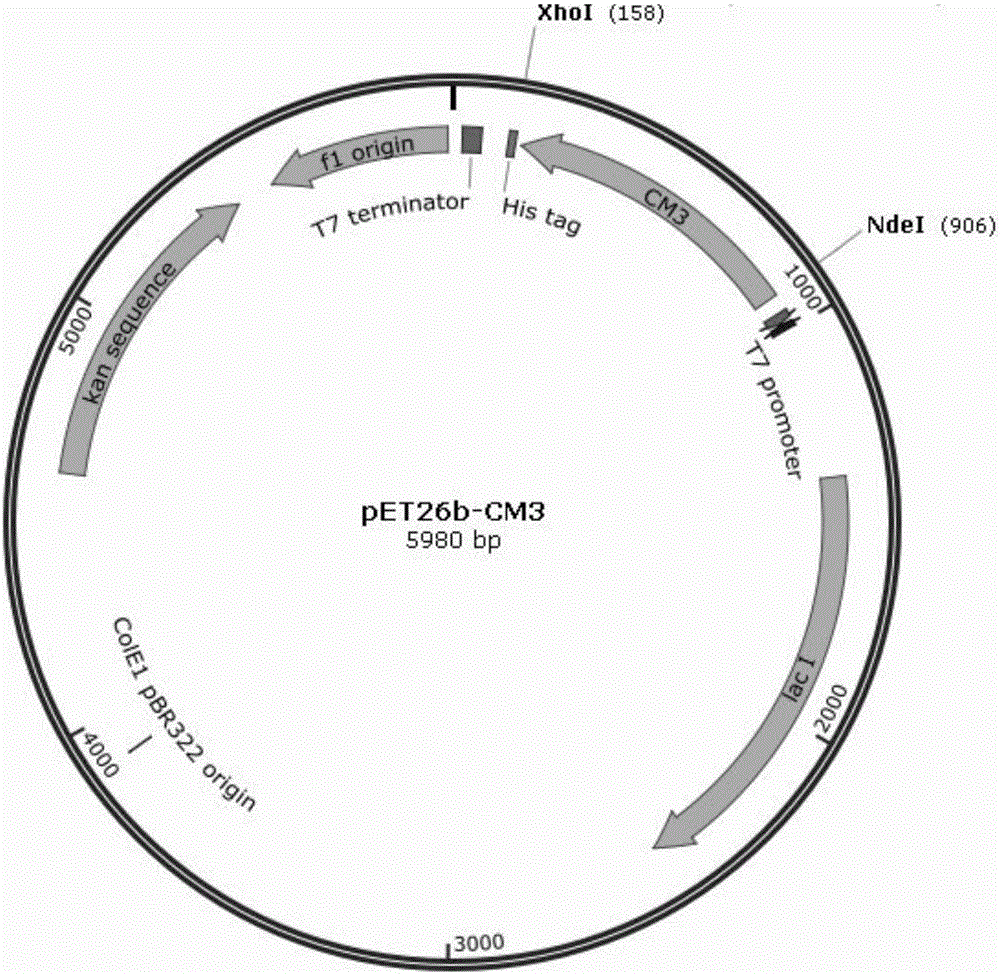
[0027] (1) Synthesis of the gene encoding carbonyl reductase: according to the original gene sequence of carbonyl reductase CM3 (genebankSequence ID: ABB91667.1), the codon optimization of the original gene sequence was carried out according to the codon analysis table of Escherichia coli and the synthesis of the target gene was carried out. Total synthesis, gene synthesis entrusted to Shanghai Jierui Biotechnology Co., Ltd., the obtained coding gene sequence is shown in SEQ ID NO.2, and the amino acid sequence of the translated carbonyl reductase is shown in SEQ ID NO.1. Both ends of the synthesized coding gene were cut with NdeI and XhoI restriction sites respectively and connected to pET26b(+) to obtain the recombinant expression vector pET26b-CM3 (see figure 1 ).
[0028] (2) Expression of carbonyl reductase:
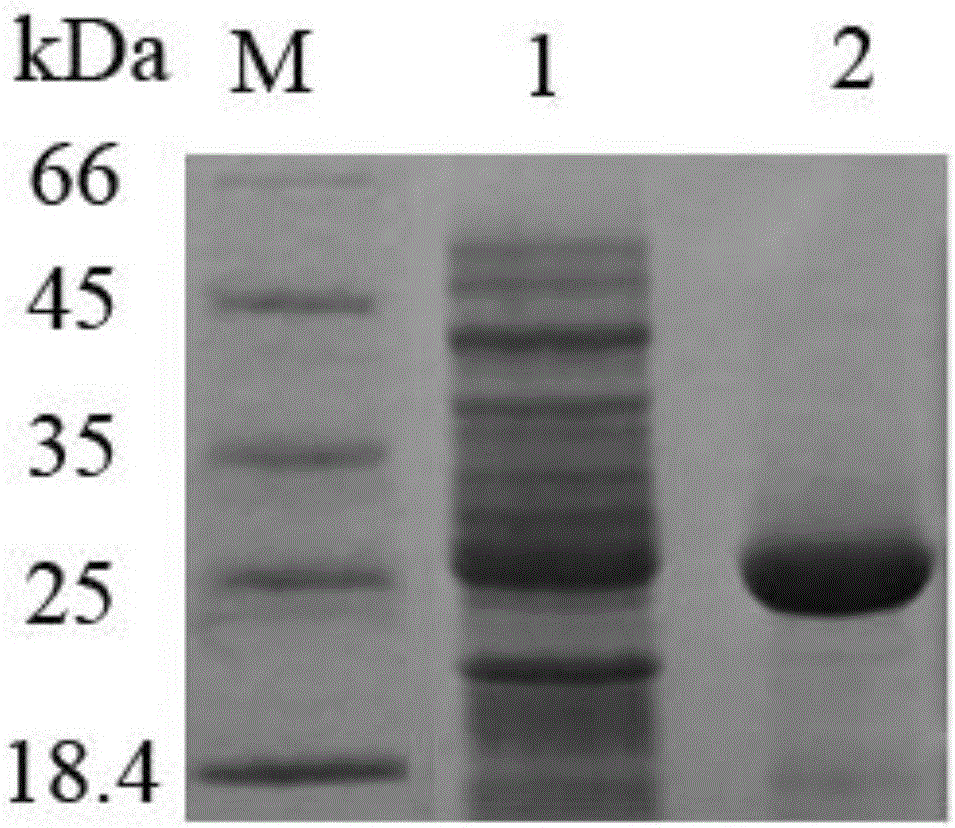
[0029] Prepare Escherichia coli BL21(DE3) competent cells, transfer the recombinant expression vector pET26b-CM3 to the expression strain BL21(DE3) by the heat sho...
Embodiment 2
[0034] The difference between this embodiment and the embodiment lies in step (4):
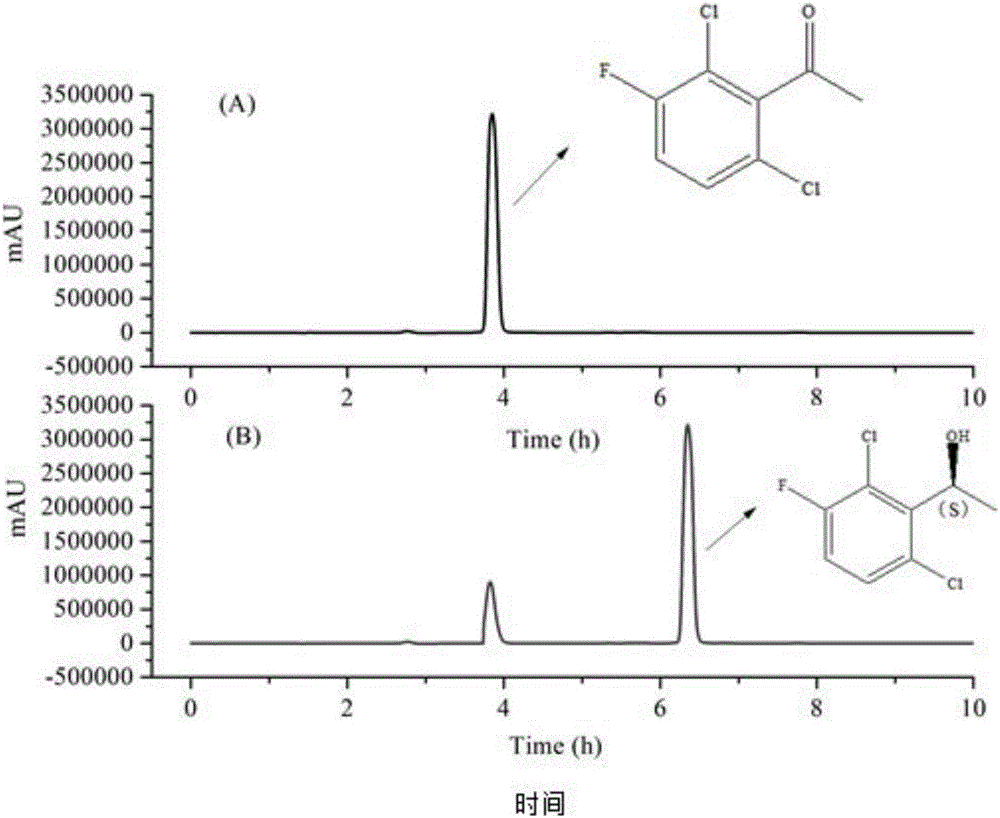
[0035] 100 g of the substrate 2',6'-dichloro-3'-fluoroacetophenone was added to a 2.5 L reactor containing 850 mL of phosphate buffer (0.1 M, pH 7.0), followed by 150 mL of 100 mL of isopropyl Alcohol, 50mg NAD + , 10 g of carbonyl reductase in 0.1 M phosphate buffer (pH 7.0) was added to the reactor. The reaction was stirred magnetically at 30 °C and the pH of the reaction was adjusted with base to maintain it at around 7.0. Thin-layer chromatography was used to qualitatively detect the consumption of substrate and the formation of product. It was detected by HPLC that the substrate was completely consumed, and NaCl was added to saturation, extracted three times with ethyl acetate, and the combined organic phases were extracted. Anhydrous sodium sulfate was used to remove water, suction filtered, and concentrated under reduced pressure to obtain an oily liquid with a chiral ee value greate...
Embodiment 3
[0037] The difference between this embodiment and the embodiment lies in step (4):
[0038] 80 g of the substrate 2',6'-dichloro-3'-fluoroacetophenone was added to a 2.5 L reactor containing 850 mL of phosphate buffer (0.1 M, pH 7.0), followed by 150 mL of 70 mL of isopropyl Alcohol, 80mg NAD + , 3 g of carbonyl reductase in 0.1 M phosphate buffer (pH 7.0) was added to the reactor. The reaction was stirred magnetically at 30 °C and the pH of the reaction was adjusted with base to maintain it at around 7.0. Thin-layer chromatography was used to qualitatively detect the consumption of substrate and the formation of product. It was detected by HPLC that the substrate was completely consumed, and NaCl was added to saturation, extracted three times with ethyl acetate, and the combined organic phases were extracted. Anhydrous sodium sulfate was used to remove water, suction filtered, and concentrated under reduced pressure to obtain an oily liquid with a chiral ee value greater t...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap