A blue light excitation mn4+ doped oxyfluoride red phosphor and preparation method thereof
A technology of oxyfluoride and phosphor, which is applied in the field of oxyfluoride red phosphor and its preparation, can solve the problems of expensive phosphor and achieve high luminous efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: take by weighing 0.664 g and Nb 2 o 5 Dissolve in 5ml hydrofluoric acid (40wt%), stir at room temperature for 60 minutes until completely dissolved, add 0.062g potassium hexafluoromanganate to the solution and react for 30 minutes; then add 0.608g cesium fluoride solid and continue stirring for 50 minutes. The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product Cs 2 Nb 5 :Mn 4+ .
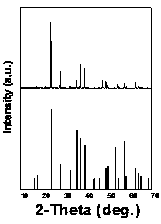
[0020] The XRD diffraction pattern of this fluorescent powder is attached figure 1 As shown, the diffraction peak of the sample is consistent with the standard card JCPDS 45-0940 (Cs 2 Nb 5 ) are completely consistent, and no diffraction peaks of any heterogeneous phases are observed, which indicates that our synthesized samples have a single crystal phase.
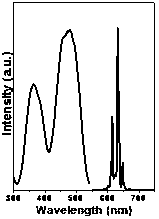
[0021] attached figure 2 Shown are the room temperature excitation spect...
Embodiment 2
[0023] Embodiment 2: take by weighing 0.664 g and Nb 2 o 5 Dissolve in 5 ml of hydrofluoric acid (40wt%), stir at room temperature for 40 minutes until completely dissolved, add 0.032 g of potassium hexafluoromanganate to the solution and react for 40 minutes; then add 0.208 g of rubidium fluoride solid and continue stirring for 60 min . The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product Rb 2 Nb 5 :Mn 4+ .
[0024] attached Figure 4 Shown is the XRD diffraction pattern of this phosphor, which is the same as the standard card JCPDS 43-0398 (Rb 2 Nb 5 ) consistent, our synthesized samples have a single crystal phase.
[0025] attached Figure 5 Shown are the room temperature excitation spectrum (monitored at 632 nm) and emission spectrum (excited at 465 nm) of the sample. The sample has strong broadband excitation in both u...
Embodiment 3
[0027] Embodiment 3: take by weighing 0.664 g and Nb 2 o 5Dissolve in 5 ml of hydrofluoric acid (40wt%), stir at room temperature for 40 minutes until completely dissolved, add 0.032 g of potassium hexafluoromanganate to the solution and react for 40 minutes; then add 0.084 g of sodium fluoride solid and continue stirring for 60 min . The resulting precipitate was washed 3 times with anhydrous acetic acid and anhydrous methanol, and finally dried in a vacuum oven for 24 hours. The orange-red powder obtained was the final product Na 2 Nb 5 :Mn 4+ .
[0028] attached Figure 7 Shown is the XRD diffraction pattern of this phosphor, which is consistent with the standard card JCPDS 77-1423 (Na 2 Nb 5 ) consistent, our synthesized samples have a single crystal phase.
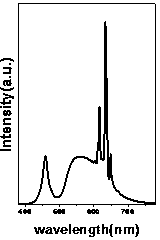
[0029] attached Figure 8 Shown are the room temperature excitation spectrum (monitored at 630 nm) and emission spectrum (excited at 460 nm) of the sample. The sample has strong broadband excitation in both ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com