Preparation method and application of benzoquinoline ratio-type near-infrared fluorescent molecular probe capable of being used for fluorine ion detection
A fluorescent molecular probe, benzoquinoline technology, applied in the field of chemical analysis and detection, can solve the problems of large fluorescence detection error, short probe emission wavelength, poor biocompatibility, etc., and achieve low cytotoxicity, low cost, and specificity strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of compound 1
[0027] 10-Hydroxybenzo[H]quinoline (585.6 mg, 2.5mmol) and hexamethylimine (1680 mg, 10mmol) were added in a 25mL round bottom flask, then 25mL trifluoroacetic acid was added to the reaction system, and the temperature was raised to 90 o C is heated to reflux. After the reaction is over, remove the reaction, let it cool and pour it into 500mL ice water, adjust the pH to about 6 with 1 N NaOH solution, a large amount of precipitate precipitates, filter, collect and wash the filter cake, and vacuum dry Afterwards, it was purified by column chromatography to obtain 512.4 mg of the product with a yield of 76.5%.
[0028] Synthesis of Compound 2
[0029] Compound 1 (446.4 mg, 2 mmol) was added in 10 mL of ethanol in a 25 mL round-bottomed flask, and after fully dissolving, 2-picoline salt (702 mg, 2.75 mmol) and 1 mmol of piperidine were added to the reaction system, and the temperature was raised to 90 o C heated to reflux. After the reaction, ...
Embodiment 2
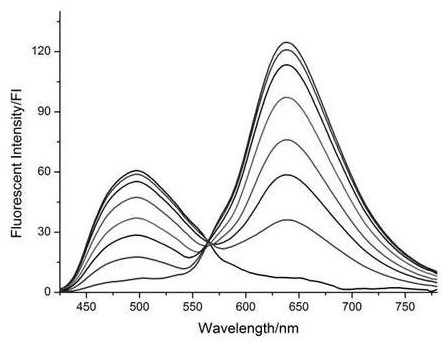
[0033] The experiment of the relationship between the fluorescence intensity of fluorescent probe and the concentration of fluoride ion
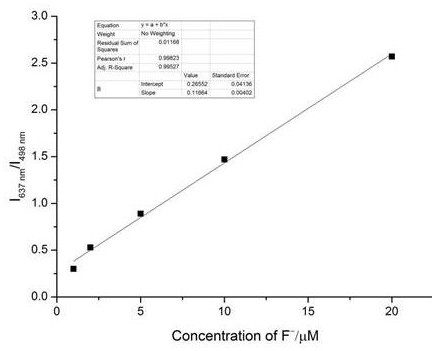
[0034] Accurately weigh 554.5 mg of the target fluorescent molecular probe with a purity of more than 99% prepared in Example 1 and dissolve it in an acetonitrile solution to prepare a 1 mM probe mother solution. Take the fluorescent probe mother solution, divide it into 8 groups, each group is 10mL, add different concentrations of fluoride ion solution, adjust the concentration of the probe molecule in the solution to 10μM, and the concentration of fluoride ion is 1μM, 2μM, 5μM, 10μM respectively , 20 μM, 30 μM, 40 μM, 50 μM. After incubation at room temperature for 20 min, the fluorescence spectra of different systems were tested in 10 mm cuvettes. The results are attached figure 1 It shows that after adding fluoride ions, the fluorescence intensity at 498 nm of the detection system gradually weakens, and the fluorescence intensity at 63...
Embodiment 3
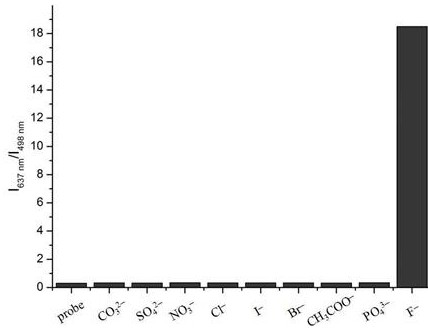
[0036] Selectivity Experiment of Fluorescent Probes to Fluoride Ions
[0037] Take the fluorescent probe solution and divide it into 10 groups, 10mL in each group, among which 1 group does not add the analyte, and 9 groups add CO 3 2 -, SO 4 2 -, NO 3 -, Cl-, I-, Br-, CH 3 COO-, PO 4 3 -, F-solutions, so that the concentration of probe molecules in each group of solutions is 10 μM, the concentration of F- is 50 μM, and the concentration of other anions is 100 μM. After incubating at room temperature for 20 min, test the fluorescence spectra of different systems in 10 mm cuvettes, record the fluorescence intensities at 637 nm and 498 nm and calculate the I 637 nm / I 498 nm value. The results are attached figure 2 Display: It is found that only when fluoride ion is added, I 637 nm / I 498 nm There was a significant change in the value of , but when other test substances were added, there was only a weak or no change in the fluorescence. It shows that the fluorescent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com