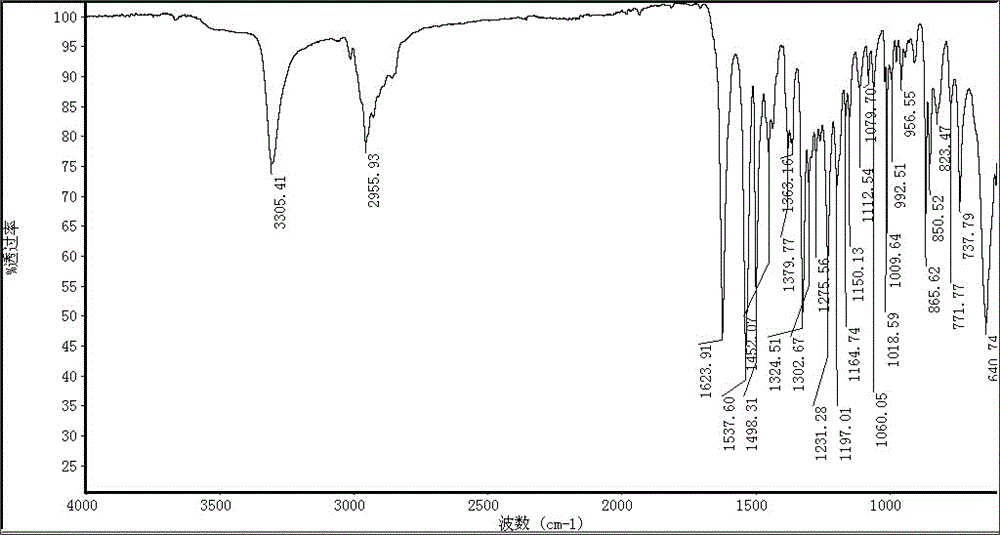
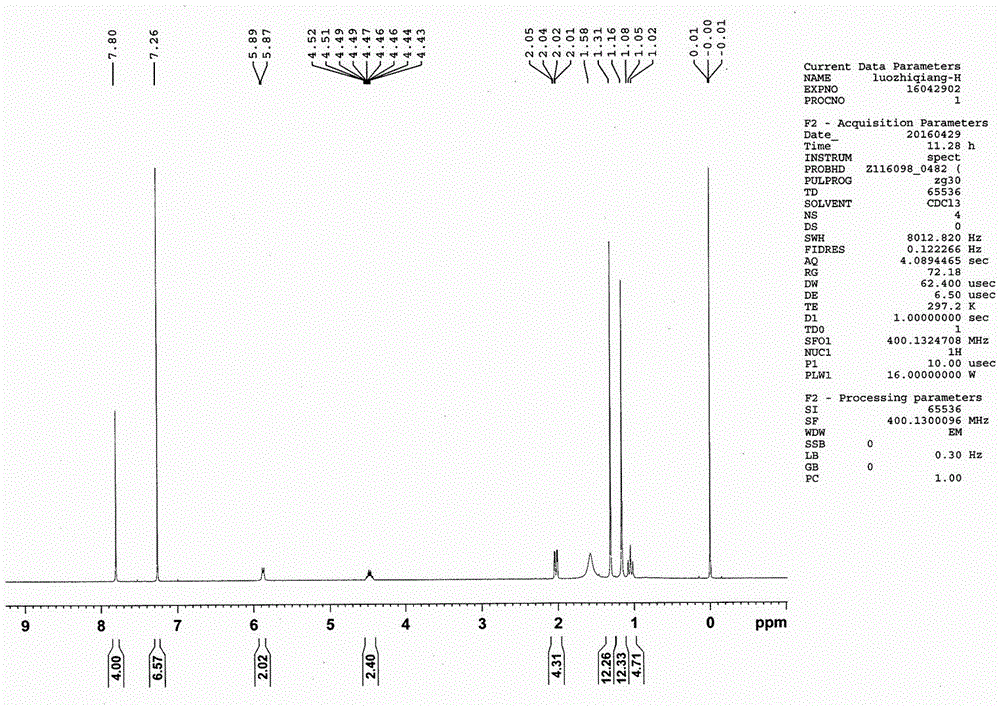
Synthesis of stabilizing agent with polyamide
A polyamide and stabilizer technology, applied in the field of synthesis of polyamide stabilizer N,N'-bis-1,4-phthalamide (I), can solve the problem of no preparation instructions, no literature on synthesis and physical and chemical properties Issues such as reports, no synthesis instructions and synthesis examples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 6.0 kg of dimethyl terephthalate and 22.0 kg of 2,2,6,6-tetramethyl-4-aminopiperidine into the reactor, and close all the inlets and outlets of the reactor. First react at 190°C for 2 hours, then raise the temperature to 230°C for 5 hours. Cool down to crystallize, and recover excess 2,2,6,6-tetramethyl-4-aminopiperidine by filtration. Then wash the generated N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,4-benzenedicarboxamide with water, filter and dry to obtain 12.3 kg Square flaky white crystal product with a yield of 89.9%.
Embodiment 2
[0019] Add 6.6 kg of diethyl terephthalate and 24.0 kg of 2,2,6,6-tetramethyl-4-aminopiperidine into the reactor, and close all the inlets and outlets of the reactor. First react at 190°C for 2 hours, then raise the temperature to 230°C for 6 hours. Cool down to crystallize, and recover excess 2,2,6,6-tetramethyl-4-aminopiperidine by filtration. Then the resulting N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,4-benzenedicarboxamide was washed with water, filtered and dried to obtain 11.5 kg Square flaky white crystal product with a yield of 87.5%.
Embodiment 3
[0021] Add 6.0 kg of dimethyl terephthalate and 22.0 kg of recovered and purified 2,2,6,6-tetramethyl-4-aminopiperidine into the reactor, and close all inlets and outlets of the reactor. First react at 190°C for 2 hours, then raise the temperature to 230°C for 5 hours. Cool down to crystallize, and recover excess 2,2,6,6-tetramethyl-4-aminopiperidine by filtration. Then wash the generated N,N'-bis(2,2,6,6-tetramethyl-4-piperidinyl)-1,4-benzenedicarboxamide with water, filter and dry to obtain 12.1 kg Square flaky white crystal product with a yield of 88.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com