L-p-nitrophenylalanine amide dipeptide derivative and its preparation method and application
A technology of nitrophenylalanine amide and peptide derivatives, which is applied in the field of drug synthesis and can solve problems such as indigestion, inability to effectively improve diabetes lipid metabolism disorder, liver toxicity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0037] In order to make the object, technical solution and beneficial effect of the present invention clearer, preferred embodiments of the present invention will be described in detail below.
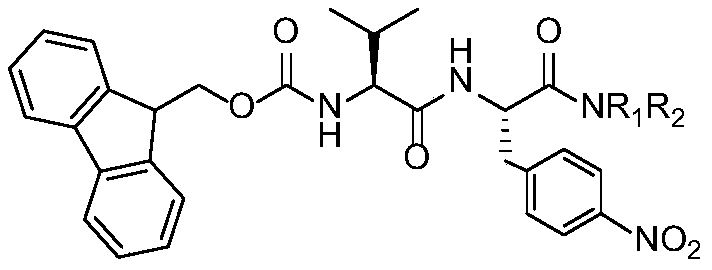
[0038] 1. Synthesis of L-p-nitrophenylalaninamide dipeptide derivatives
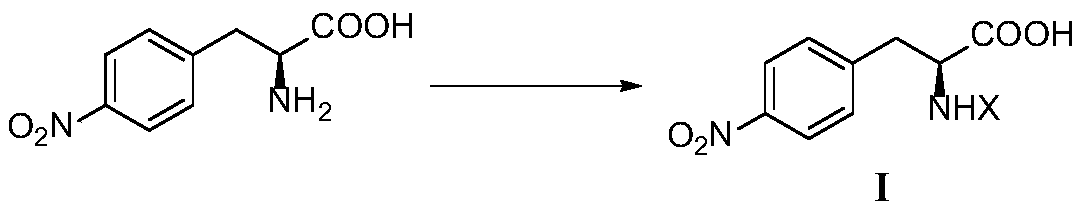
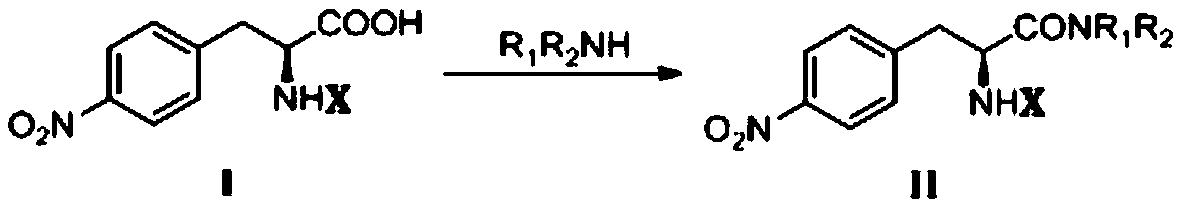
[0039] 1. Synthesis of Intermediate I
[0040]
[0041] Under ice bath, add 20mmol of L-p-nitrophenylalanine and saturated Na to the reaction flask 2 CO 3 Aqueous solution 50mL, stirred in an ice bath until dissolved, slowly drop into the dissolved (Boc) 2 O 24mmol of acetone solution 10mL, after stirring for 1h, transfer to a 35-55°C water bath to stir the reaction, keep the pH of the reaction solution at 8-9, monitor by thin-layer chromatography (TLC) until the reaction is completed. Under the condition of pH about 10, the reaction solution was extracted with diethyl ether (30mL×2), the pH of the aqueous phase was adjusted to 3-5 with 3N HCl, and then extracted with ethyl acetate (EA) (30mL×3). phase, wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com