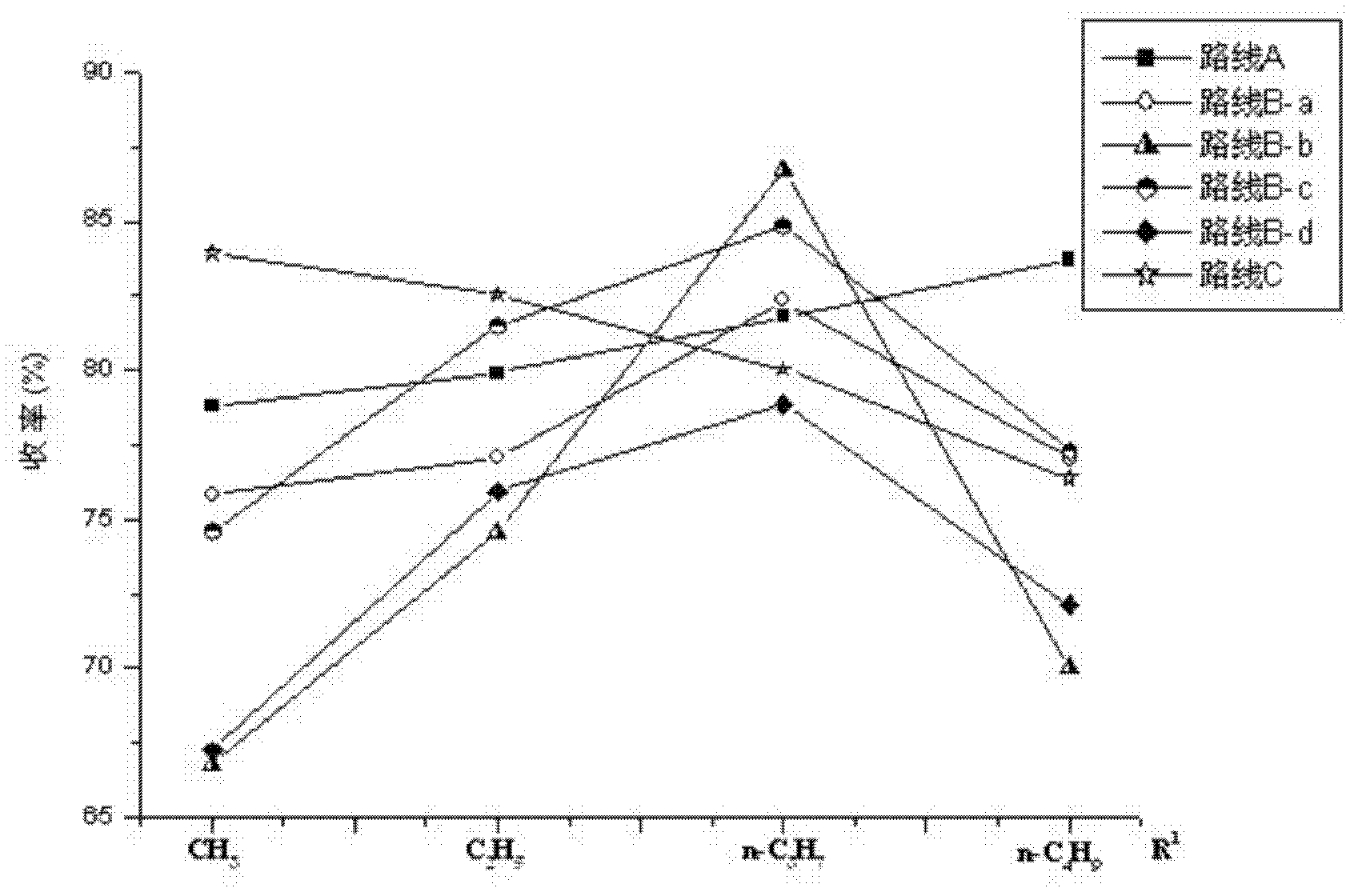
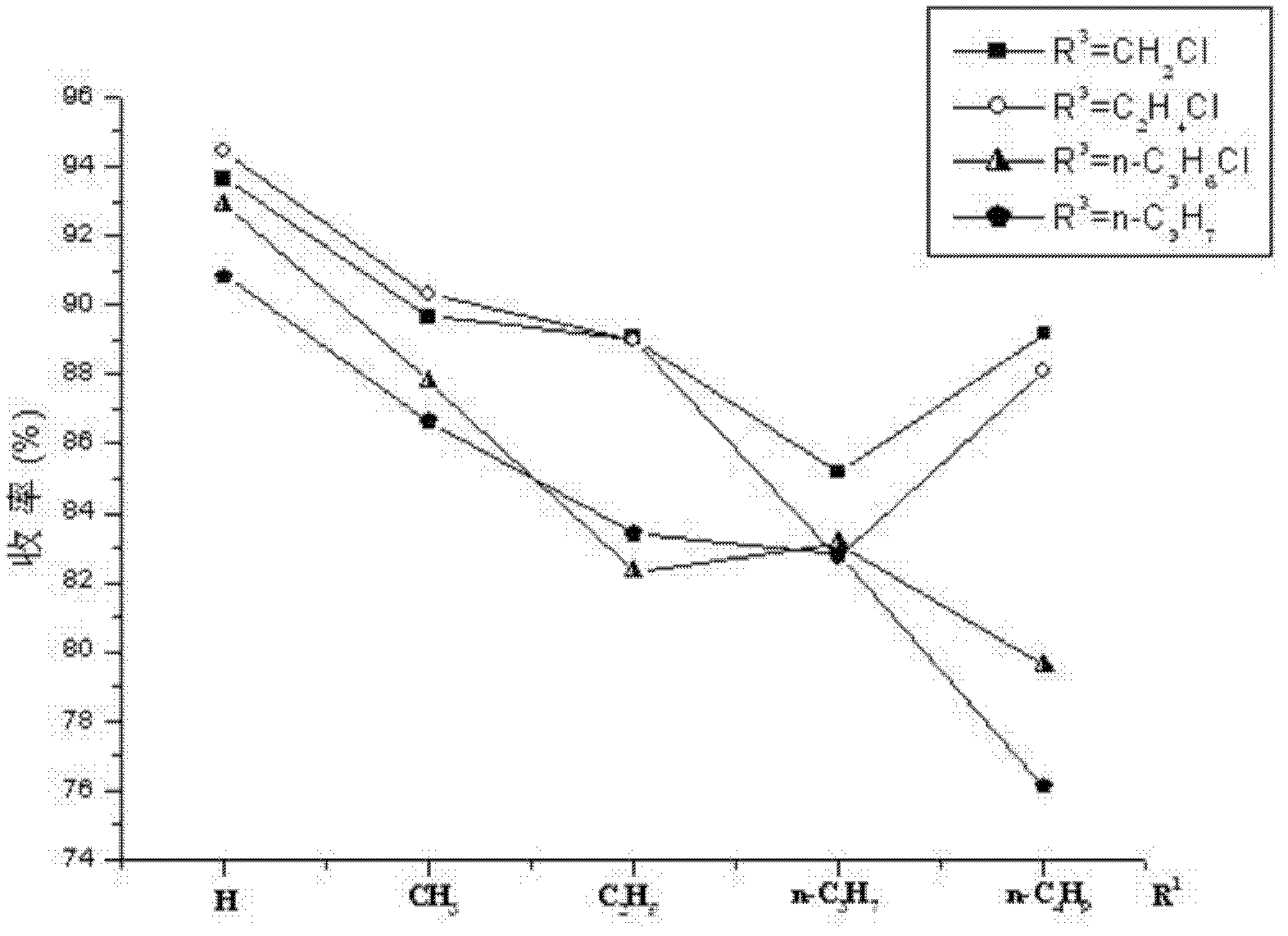
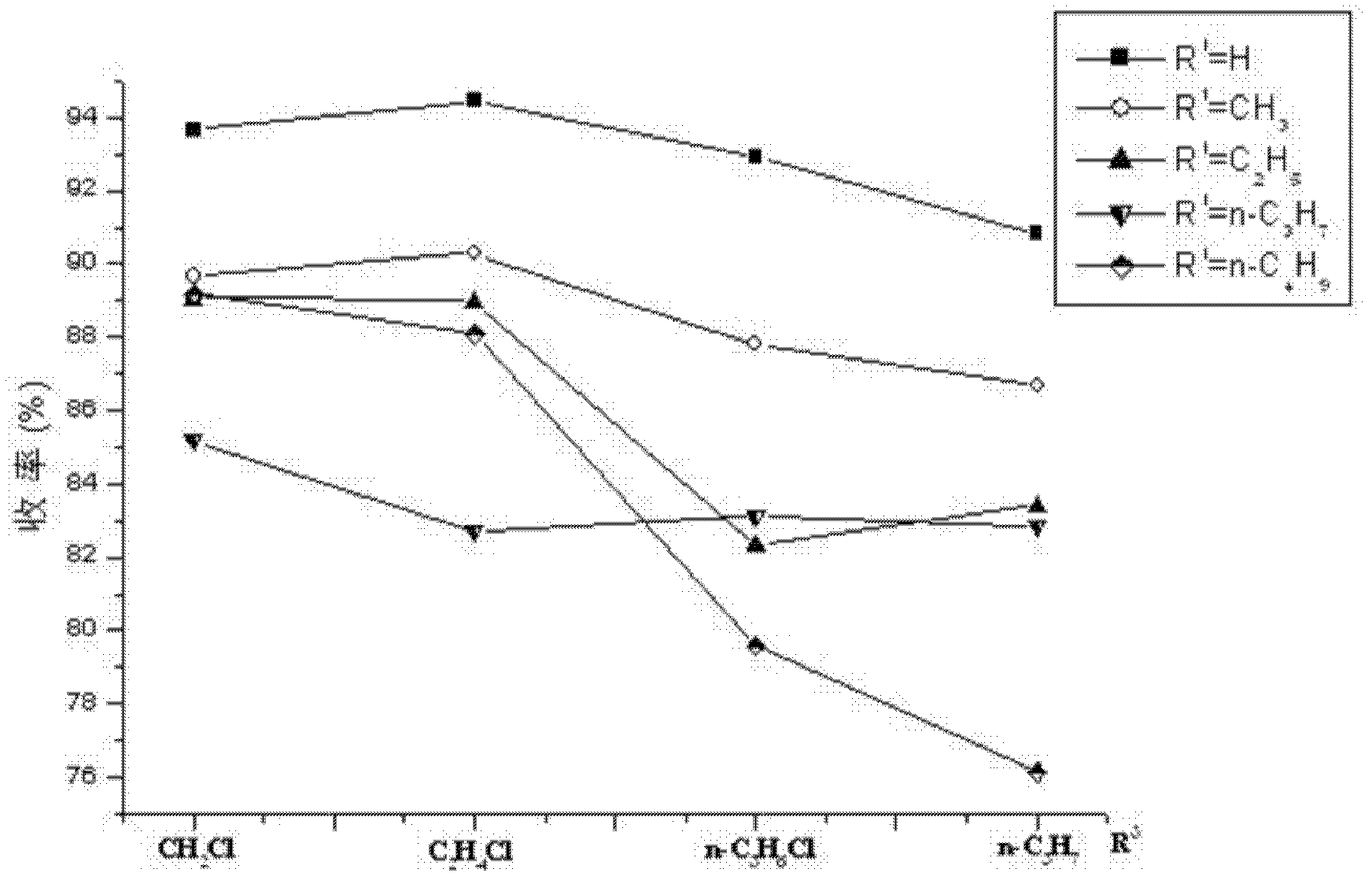
4-(4-aminobenzene sulfonamide) phenylacetic acid derivative and preparation method and application thereof
A technology of aminobenzenesulfonamide group and phenylacetic acid, which is applied in the fields of chemistry and pharmacy, can solve the problems of hypoglycemia, elevated cholesterol level, can not effectively improve diabetes lipid metabolism disorder, etc., and achieves the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the preparation of intermediate IM1
[0051]
[0052] Add p-aminophenylacetic acid (4-APA), K 2 CO 3 and an appropriate amount of water, stir to dissolve, cool in an ice bath, add dropwise an acetone solution of p-acetamidobenzenesulfonyl chloride (ASC), 4-APA, K 2 CO 3 The molar ratio of ASC and ASC is 1:2.1:1.2-1.5. After the dropwise addition, the ice bath is removed, the reaction is stirred at room temperature, and the reaction progress is monitored by thin layer chromatography (TLC). After the reaction, the acetone was removed by rotary evaporation under reduced pressure to obtain a light yellow turbid liquid, which was filtered with suction, the filter cake was washed with water, and dried to obtain a crude product. Put the crude product in 20mL 2N KOH, stir and dissolve for 0.5h, filter with suction, wash the filter residue with water, combine the lotion with the filtrate, cool in an ice bath, slowly add concentrated HCl dropwise, a large amou...
Embodiment 2
[0056] Embodiment 2, the preparation of intermediate IM2
[0057]
[0058] Add IM1 and a certain concentration of KOH solution to the reaction flask in sequence, stir to dissolve, raise the temperature to 60-65°C and control the temperature to stir the reaction, and monitor the reaction progress by TLC. After the reaction was completed, cool in an ice bath, slowly add concentrated HCl dropwise, a large amount of solids precipitated, adjust the pH=4 to 5 (subject to no more solid precipitation), filter with suction, wash the filter cake with water, and dry at room temperature to obtain the crude product . The crude product was dispersed with 0.2N HCl, filtered with suction, the filter cake was washed with water, and dried in vacuum to obtain the intermediate IM2. Some experimental conditions and results are shown in Table 2.
[0059] Preparation conditions and results of Table 2IM2
[0060]
[0061] IM2 4-(4-aminobenzenesulfonamido)phenylacetic acid: m.p.121.4~122.7℃; ...
Embodiment 3
[0062] Embodiment 3, the preparation of 4-(4-aminobenzenesulfonamido) phenylacetic acid derivative TM1
[0063]
[0064] Add IM2 and K to the reaction flask in sequence 2 CO 3 0.70g (5mmol) and 8mL of acetone, stir to dissolve, cool in an ice bath, add acid chloride R dropwise 3 After COCl was added, the reaction was stirred under temperature control, and the reaction progress was monitored by TLC. After the reaction, acetone was removed by rotary evaporation, an appropriate amount of water was added to stir, and 2N HCl was added dropwise to adjust the pH to 2-3 (subject to the fact that no solids were precipitated), left to stand, suction filtered, the filter cake was washed with water, and dried at room temperature to obtain Crude. The crude product was recrystallized from mixed solvents such as DCM-petroleum ether and EA-petroleum ether (the sample for analysis was further purified by column chromatography) to obtain compound TM1. Part of the experimental conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com