Parenteral glucagon formulations
一种胰高血糖素、肠胃外的技术,应用在可注射液体组合物领域,能够解决胰高血糖素降解困难、未曾报道等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
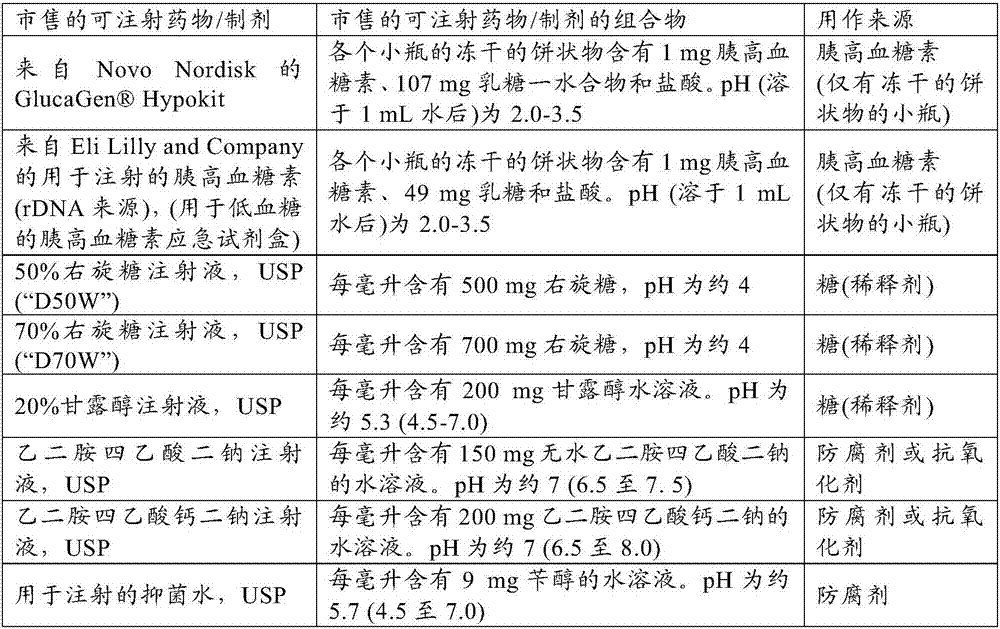
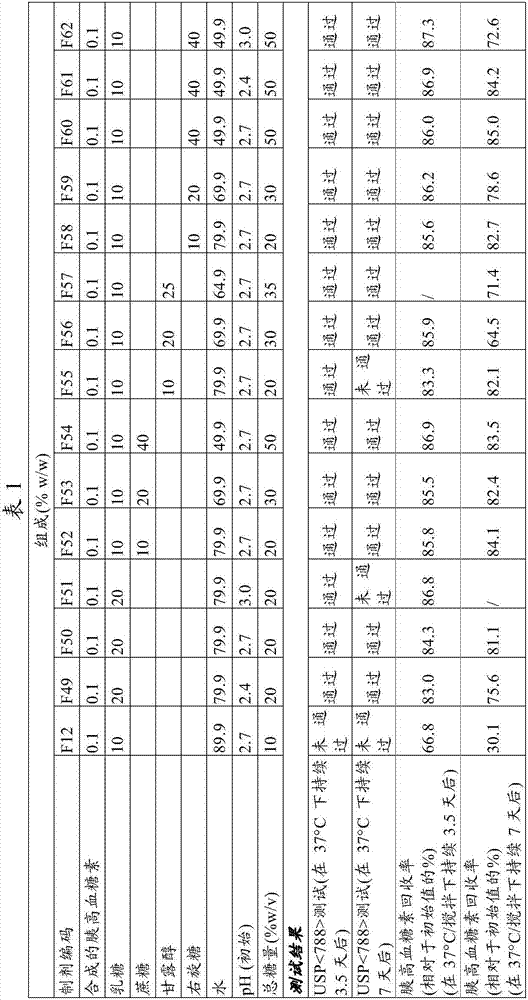
[0138] The purpose of this study was to determine the effect of high sugar concentrations (>10% w / v) on glucagon gelation. For each test composition, a lyophilized cake containing 1 mg glucagon and 100 mg lactose was prepared to have the same The same lyophilized cake composition in the Hypokit, except that the glucagon in these lyophilized cakes was produced synthetically rather than by recombinant DNA methods. Each lyophilized cake was dissolved in about 1 mL of sugar solution to obtain the final liquid composition as shown in Table 1. For comparison, F12 was prepared to have the same Mixing of the 2 vials supplied by Hypokit produces a final solution of the same composition.
[0139] Each solution was filled into an insulin pump reservoir cartridge (Medtronic, Inc. example reservoir #MMT-332A), placed on a shaker platform in a 37°C incubator and subjected to continuous agitation at 100 RPM. After 0, 3.5, and 7 days, a portion of the solution was removed and tested for ...
Embodiment 2
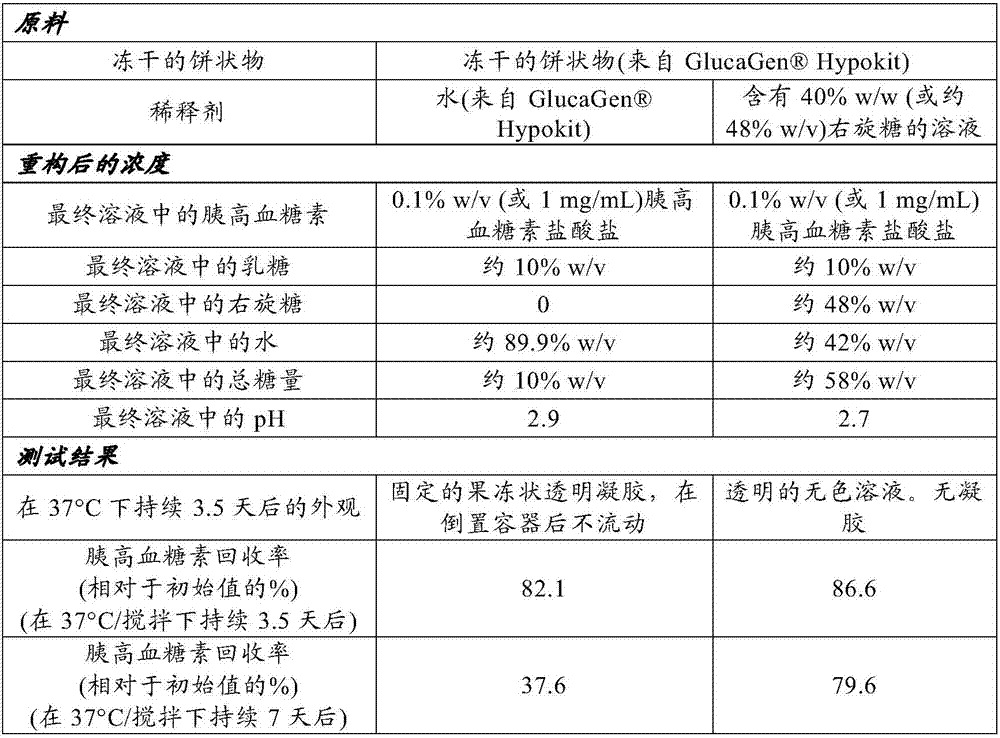
[0143] The purpose of this study was to compare the rates of gelation and chemical degradation of glucagon in solutions prepared by diluting the glucagon from Lyophilized cakes of Hypokit (Novo Nordisk) were dissolved to prepare. These reconstituted solutions were tested using the same procedure as in Example 1. The final solution composition and test results are shown in Table 2. Manufactured using recombinant DNA methods Glucagon in Hypokit freeze-dried cakes.
[0144] Table 2
[0145]
[0146] The results of the present study demonstrate that high concentrations (≥20%) of sugars or combinations of sugars significantly improve the stability of glucagon at body temperature by inhibiting gelation and chemical degradation in solutions containing Hypokit recombinant DNA produced glucagon.
Embodiment 3
[0148] The purpose of this study was to compare the rate of chemical degradation of glucagon between solutions obtained from commercially available The lyophilized cake of the Glucagon kit was prepared by dissolving. The lyophilized cake from the Glucagon for Injection (rDNA Origin) kit from Eli Lilly and Company ("Glucagon Emergency Kit for Hypoglycemia") was tested using the same procedure as Example 1 shape. The final solution composition and test results are shown in Table 3. Lyophilized cakes of the glucagon in the Glucagon Emergency Kit for Hypoglycemia were manufactured using recombinant DNA methods.
[0149] table 3
[0150]
[0151] These results indicate that high concentrations (>20% w / v) of sugar or combination of sugars significantly improved glucagon solution stability at body temperature by inhibiting chemical degradation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com