A kind of photosensitizer compound and its preparation method and application
A technology for photosensitizers and complexes, applied in the field of photosensitizer complexes and their preparation, can solve the problems of being unsuitable for the treatment of hematological tumors, losing the selectivity of the illumination area, etc., and achieves enhanced stability, improved targeting, and high safety. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing the photosensitizer complex according to the above technical solution, which includes the following steps:
[0049] 1) Mixing chitosan and hydrophobic compounds in a mass ratio of (20-30):4 for esterification reaction to obtain a grafted polymer, and removing residual hydrophobic compounds by dialysis;
[0050] 2) Dissolve the graft polymer described in step 1) in a polar solution to obtain a graft polymer solution with a concentration of 0.5 to 1.5 mg / mL, and add the photosensitizer dropwise to the graft polymer at a rate of 20 to 40 μL / min In the solution, micelles loaded with photosensitizer are obtained;
[0051] 3) The photosensitizer-loaded micelles described in step 2) are subjected to an esterification reaction with a specific antibody to obtain a photosensitizer complex.
[0052] In the present invention, the chitosan and the hydrophobic compound are mixed according to a mass ratio of (20-30):4 to carry out an ...
Embodiment 1
[0075] Construction of MEDI-551-scFv expression vector:
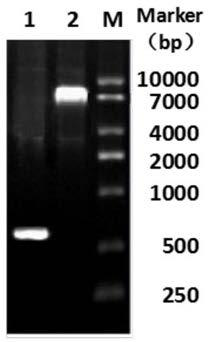
[0076] Obtain the VH sequence (shown in SEQ ID NO: 1) and VL sequence (shown in SEQ ID NO: 2) of the anti-CD19 antibody (MEDI-551) through drugbank, and then use the overlap PCR method to pass a flexible peptide ( G 4 S) 3 Connect the C-terminus of VL and N-terminus of VH to obtain the sequence of anti-CD19 single-chain antibody (MEDI-551-scFv) (shown in SEQ ID NO: 3), and add BamHI and HinderIII to the 2 ends of the sequence The restriction site was inserted into the expression vector pET-SUMO in the manner of enzyme digestion. After colony PCR verification and sequencing, the correct recombinant expression vector was screened: pET-SUMO-MEDI-551-scFv electrophoresis results are as follows figure 1 As shown, Lane1 is a single-chain antibody (MEDI-551-scFv); Lane2 is a recombinant expression vector (pET-SUMO-MEDI-551-scFv).
[0077] The recombinant expression vector was transformed into Origami 2 (DE3), and the correct expres...
Embodiment 2
[0079] Obtaining of MEDI-551-scFv protein:
[0080] The expression strain (Origami 2(DE3) / pET-SUMO-MEDI-551-scFv) was subjected to a 2L large-scale fermentation to extract its cytoplasmic protein. Since the target protein obtained by the expression vector has a his tag at the 5'end, And there is a section of SUMO restriction site afterwards, so the corresponding single-chain antibody (MEDI-551-scFv) protein was obtained by Ni column purification-restriction digestion-Ni column repurification.
[0081] Specific steps are as follows:
[0082] 1) Collect the cultured Escherichia coli and centrifuge at 8000 rpm for 30 min at 4°C to obtain bacterial pellet.
[0083] 2) Resuspend the pellet in 20 mL cell lysate (50 mM Tris-HCl, 0.5 M NaCl, 1% Triton X-100, pH 8.0) containing 0.1 mg / mL lysozyme, and sonicate for 10 min.
[0084] 3) After the above mixture was magnetically stirred at 4°C for 30 minutes, centrifuged at 14000 rpm for 30 minutes, and the supernatant was collected and filtered wit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com