18β-glycyrrhetinic acid derivatives and their applications
A derivative, C1-C4 technology, applied in the field of medicine, can solve problems such as not meeting clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
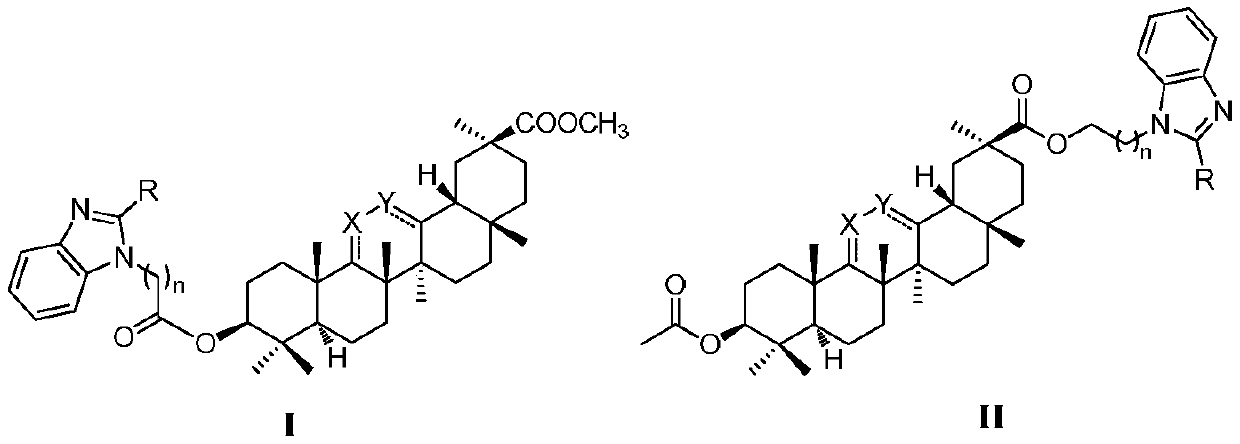
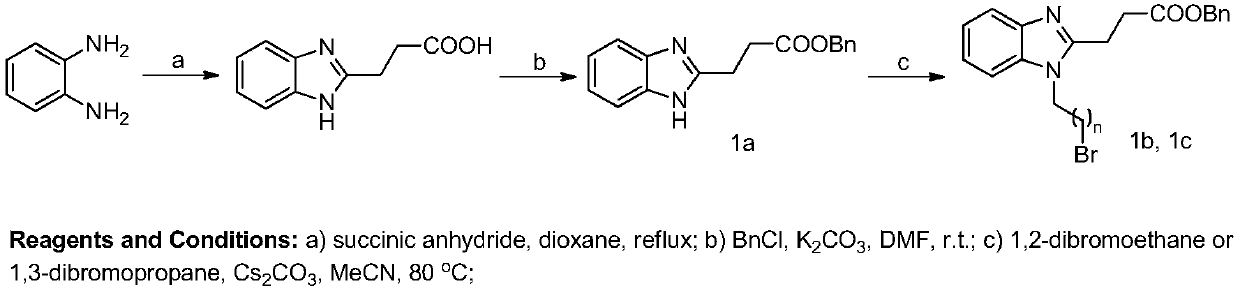
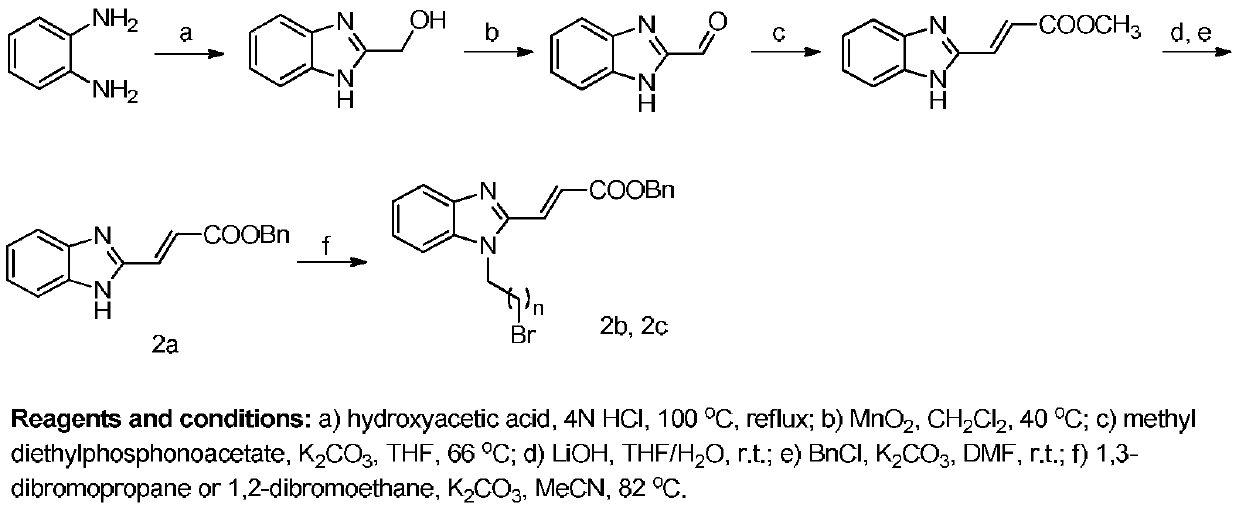
[0076] Example 1: 3-(1-(2-((11-oxo-18β-oleanane-12-ene-30-methyl carboxylate)-3β-oxo)-2-oxoethyl) Preparation of -1H-benzimidazol-2-yl)propionic acid
[0077] Step A:
[0078]
[0079] Dissolve methyl glycyrrhetinate (2.00g, 4.13mmol) in 70mL of dry dichloromethane, add triethylamine (2.00mL), add dropwise 2-chloroacetyl chloride (2.00mL, 25.36mmol) under ice-cooling, room temperature The reaction was monitored by TLC. After the reaction was completed, water was added to quench, the organic phase was separated, and the aqueous phase was extracted 3 times with dichloromethane. The organic phases were combined, dried over anhydrous sodium sulfate, and purified by column chromatography. The yield is about 68%.
[0080] Step B:
[0081]
[0082] Put o-phenylenediamine (5.00g, 46mmol) in a 250mL eggplant-shaped bottle, add 110mL of dioxane at room temperature, stir and dissolve, then add succinic anhydride (5.55g, 55.5mmol). Transfer to an oil bath, react at 80°C for 4 ...
Embodiment 2
[0092] Example 2: 3-(1-(2-((18β-Oleanane-12-ene-30-carboxylic acid methyl ester)-3β-oxygen)-2-oxoethyl)-1H-benzo Preparation of imidazol-2-yl)propionic acid
[0093] According to the preparation method of Example 1, the title compound was prepared.
Embodiment 3
[0094] Example 3: 3-(1-(2-((12-oxo-18β-oleanane-30-carboxylic acid methyl ester)-3β-oxygen)-2-oxoethyl)-1H-benzene Preparation of imidazol-2-yl)propionic acid
[0095] According to the preparation method of Example 1, the title compound was prepared.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com