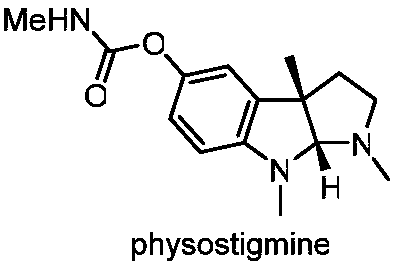
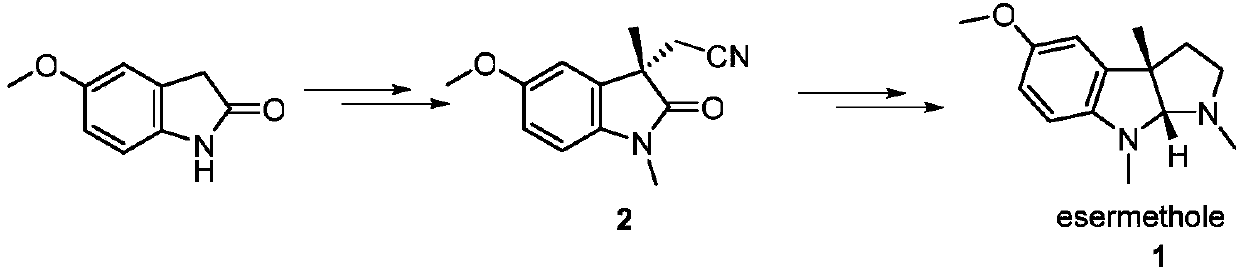
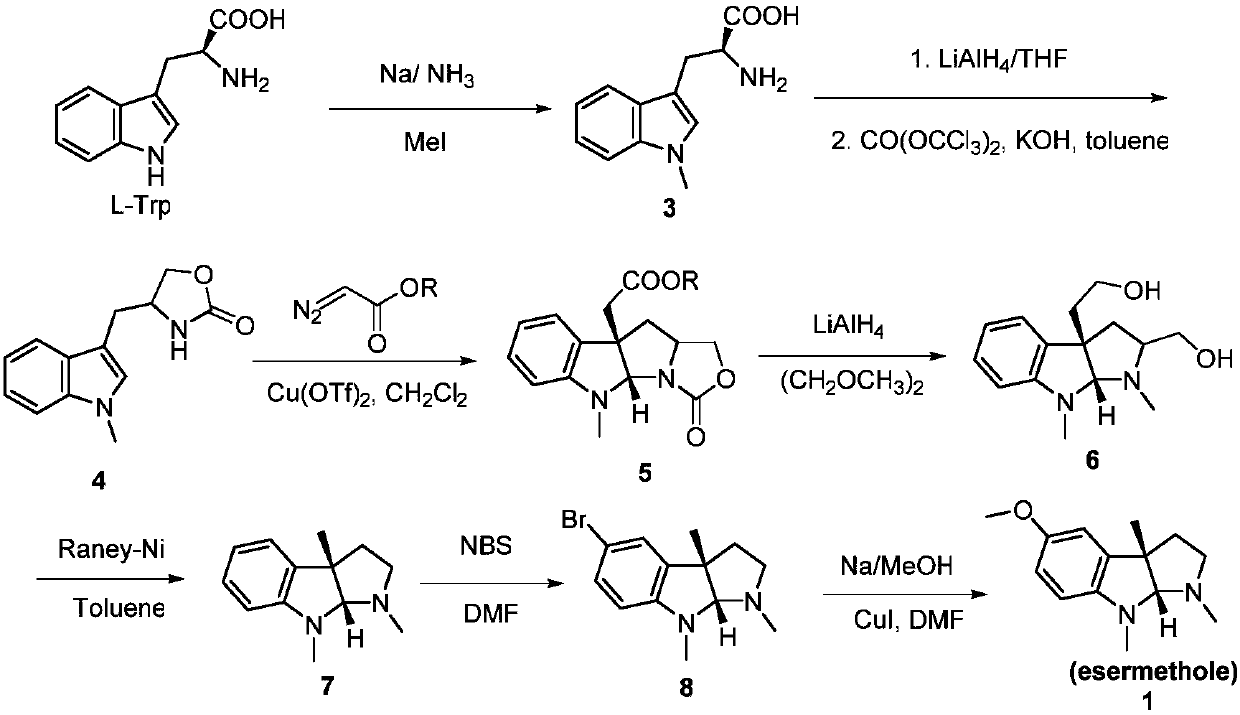
A kind of preparation method of physostigmine precursor compound
A precursor compound, physostigmine technology, applied in the direction of organic chemistry, can solve the problems of unfavorable industrial production, long synthesis steps, high equipment requirements, etc., and achieve the effect of high feasibility of industrial production, convenient operation and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0032] The preparation method of the physostigmine precursor compound of the embodiment of the present invention is given below.
[0033] step one
[0034]
[0035] Amino group protection step reaction formula
[0036] Dissolve 5-methoxytryptamine (19g, 100mmol) and 4-N,N-dimethylaminopyridine (DMAP, 1.22g, 10mmol) in acetonitrile (400mL), then add triethylamine (30.3g, 300mmol). After the reactant was stirred at room temperature for 1h, it was lowered to 0°C, then a solution of benzyl chloroformate (CbzCl, 35.8g, 210mmol) in acetonitrile (10mL) was slowly added, then raised to room temperature and stirred for 12h, and saturated chlorine was added after the reaction of the raw materials was complete. Quenched with ammonium chloride solution (100 mL), concentrated under reduced pressure to remove the acetonitrile solvent, then diluted with water (300 mL), extracted the aqueous phase with ethyl acetate (100 mL × 3), combined the organic phases, washed the organic phase with...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com