Cerebroprotein hydrolysate preparation and designing method thereof
A brain protein hydrolyzate and preparation technology, applied in the field of pharmaceutical preparations, can solve problems such as insufficient test accuracy, inability to sensitively investigate interactions, and inability to accurately find the best point, etc., to achieve the effect of prescription science
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
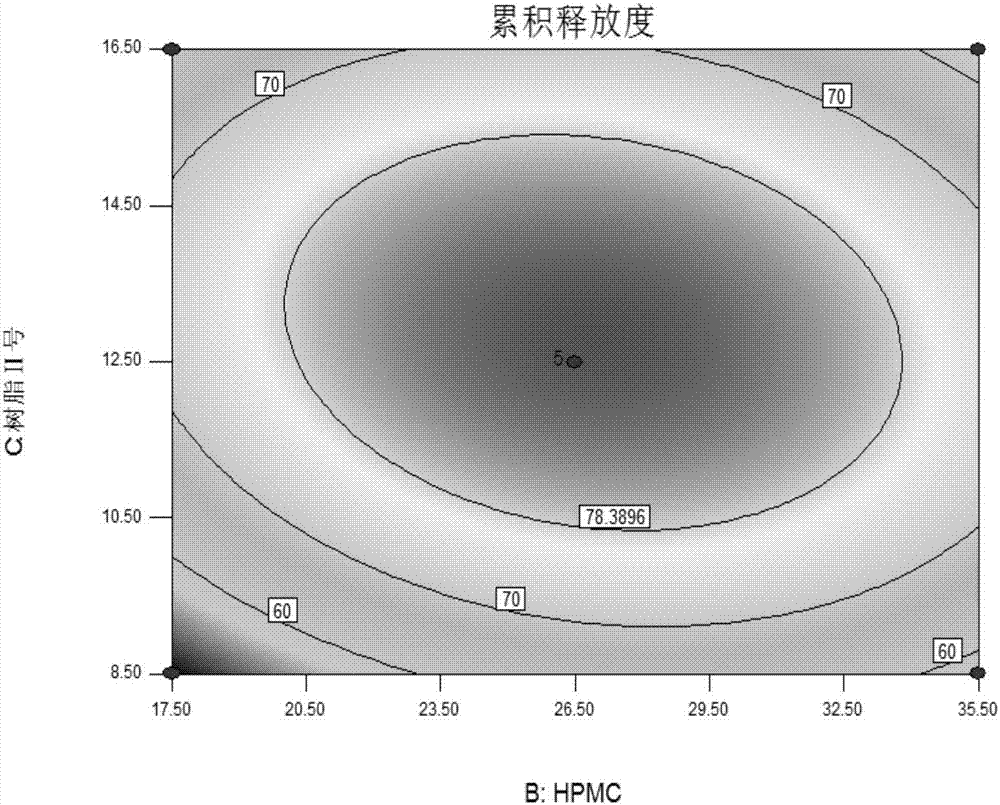
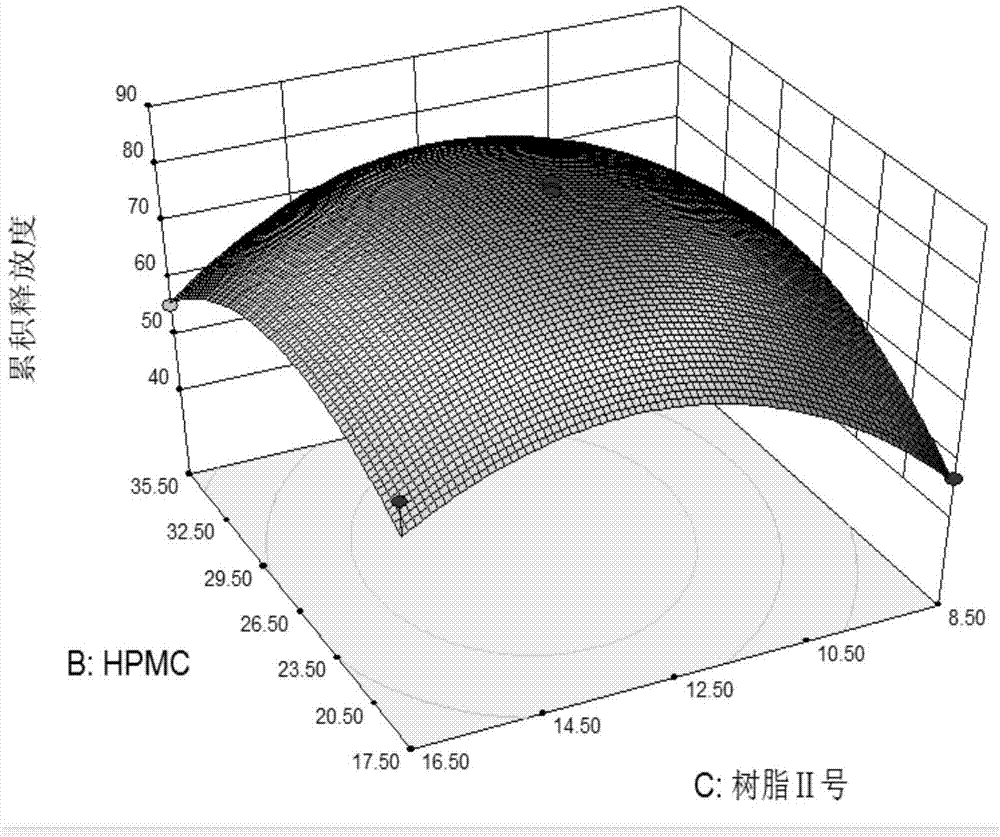
[0067] A cerebroprotein hydrolyzate preparation is designed by the following method:
[0068] 1. Choice of dosage form.
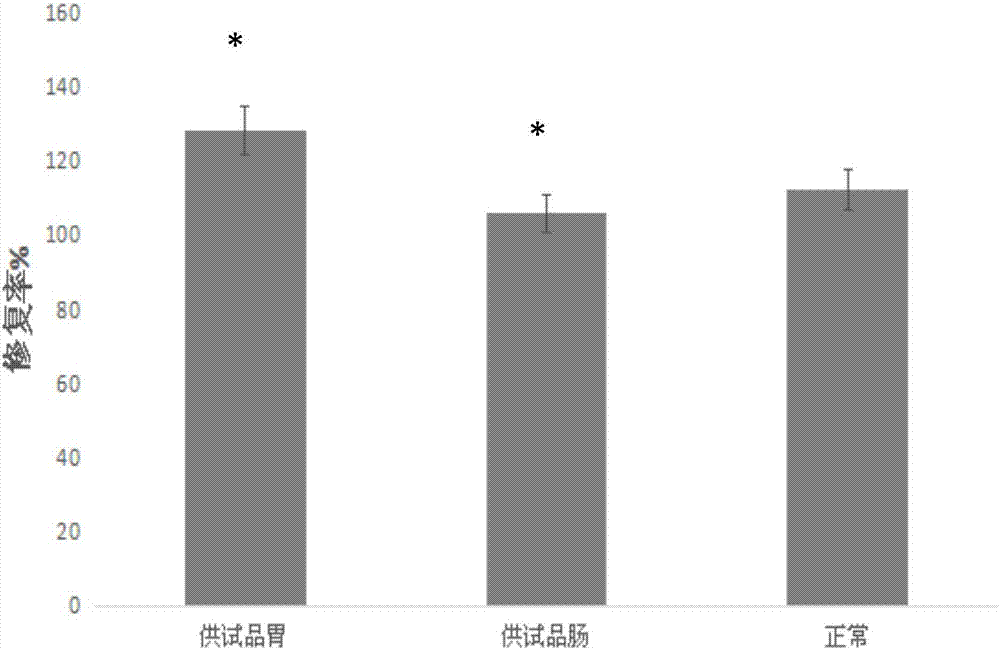
[0069] The cerebroprotein hydrolyzate was treated in artificial gastric juice and artificial intestinal juice for MTT experiment, and the dosage form was selected according to the repair rate of cerebroprotein hydrolyzate in the stomach and intestine to the oxidative damage model, as follows.
[0070] (1) Determination of the dosage of cerebroprotein hydrolyzate:
[0071] the PC 12 Cells were seeded in 96-well plates for research, and each experimental group was set up according to the following table. The specific sample addition and experimental methods were as follows:
[0072] The cell concentration was 1ml containing 1.0×10 5 The cell suspension of two cells was inoculated in the 96-well plate of the normal cell control group, the damaged cell control group, and the test product 1-3 group with different concentrations, and 100 μl was added to each w...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap