Kallidinogenase enteric coated tablet and preparation method thereof
A technology of pancreatic kininogenase and enteric-coated tablets, which can be applied in the fields of pill delivery, pharmaceutical formula, peptide/protein composition, etc., and can solve the problem that the content uniformity of finished products does not meet the requirements, the content uniformity does not meet the requirements, and the process design is unreasonable and other issues to achieve the effect of avoiding titer loss, less potency unit loss, and improving content and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
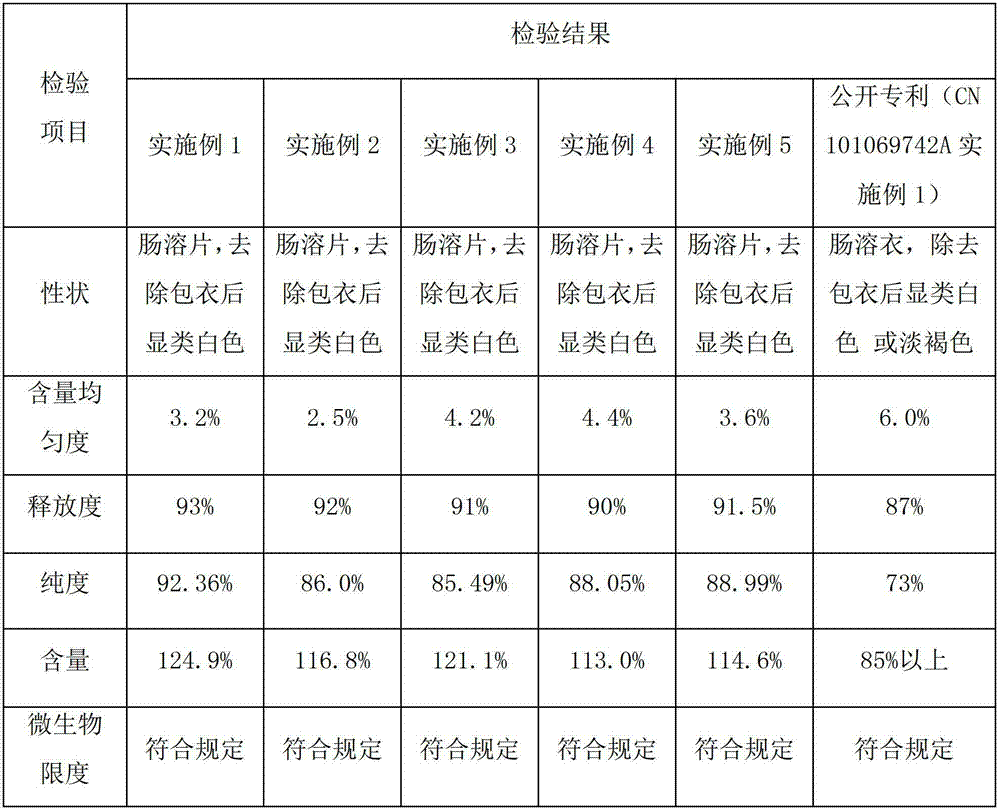
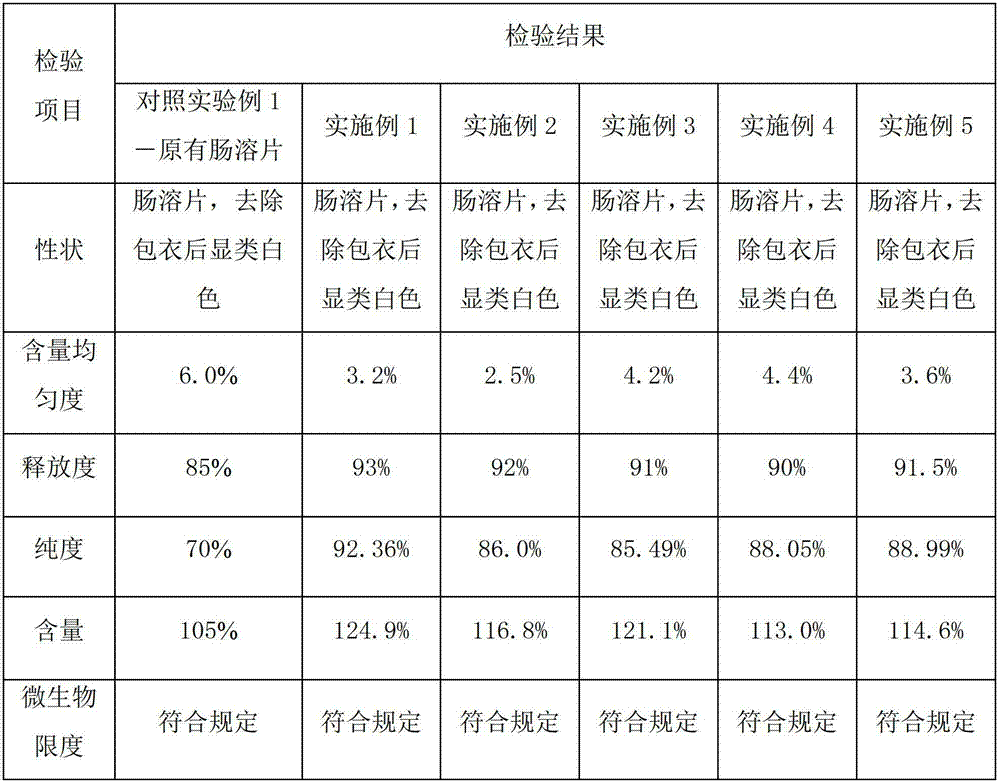
[0031] Example 1 Preparation of pancreatic kininogenase tablets
[0032] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0033] Pancreatic kininogenase: 140,000 units
[0034] Starch: 130g
[0035] Microcrystalline Cellulose: 20g
[0037] Hypromellose: 8g
[0039] Enteric Film Coating Premix (Ahua) 24g
[0040] Preparation:
[0041] 1. Preparation: Grind excipients such as pancreatic kininogenase, starch, microcrystalline cellulose, sodium carbonate, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0042] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are size...
Embodiment 2
[0048] Example 2 Preparation of pancreatic kininogenase tablets
[0049] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0050] Pancreatic kininogenase: 90,000 units
[0051] Lactose: 140g
[0052] Microcrystalline Cellulose: 15g
[0053] Sodium Alginate 25g
[0054] Hypromellose: 6g
[0056] Enteric film coating premix (Yingmao) 28
[0057] 1. Preparation: Grind excipients such as pancreatic kininogenase, lactose, microcrystalline cellulose, sodium alginate, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0058] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are sized with a 20-mesh stainles...
Embodiment 3
[0063] Example 3 Preparation of pancreatic kininogenase tablets
[0064] A pancreatic kininogenase enteric-coated tablet, every 1000 tablets includes the following components:
[0065] Pancreatic kininogenase: 120,000 units
[0066] Lactose: 120g
[0067] Sodium starch glycolate: 18g
[0069] Hypromellose: 7g
[0071] Enteric Film Coating Premix (Ahua) 20g
[0072] 1. Preparation: Grind excipients such as pancreatic kininogenase, lactose, sodium starch glycolate, calcium chloride, hypromellose, and magnesium stearate through an 80-mesh sieve.
[0073] 2. Prepare blank granules: Mix the weighed auxiliary materials according to the prescription amount, add purified water and stir evenly to make soft materials, pass through a 20-mesh sieve to prepare blank granules. Place the granules in a hot air circulation oven, and dry them with hot air below 50°C. The dried blank granules are sized with a 20-mesh stainless s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com