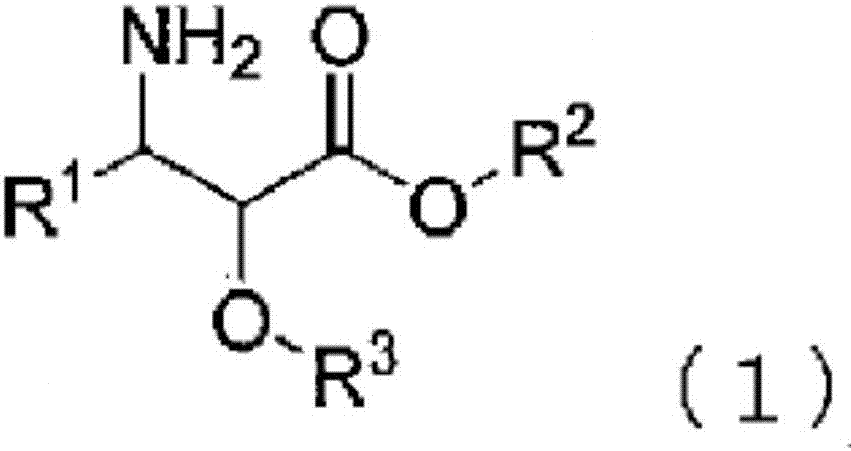
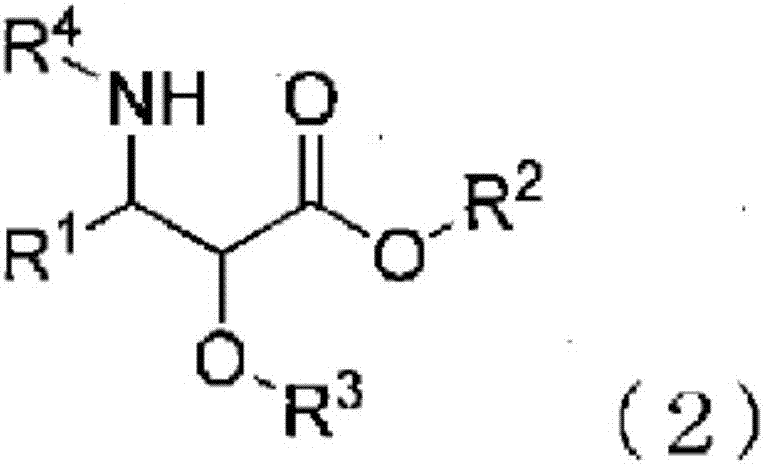
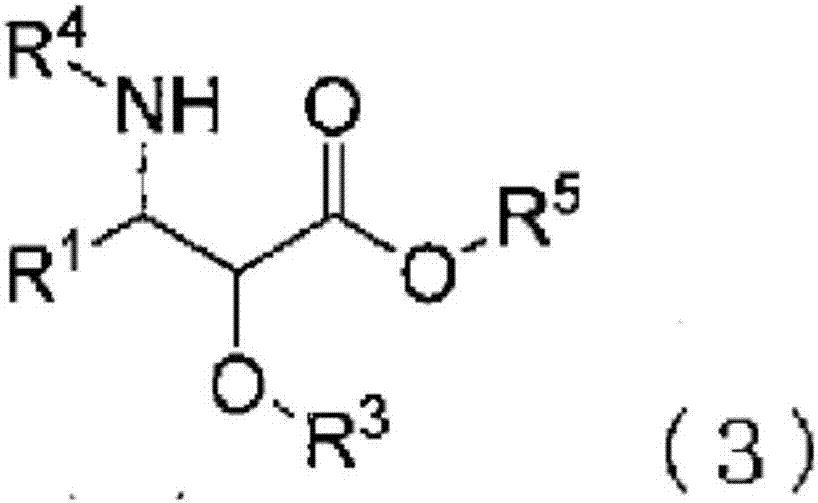
3-phenylisoserine derivative production method
A technology of phenylisoserine and its manufacturing method, which is applied in the preparation of carbamic acid derivatives, organic compounds, carboxylic acid amides, etc., and can solve the problems of solid-liquid separation, such as extremely time-consuming, low yield, and poor quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] In a 2L four-necked flask equipped with a thermometer, a condenser and a stirrer, 120 g (0.551 moles) of (2R, 3S)-3-phenylisoserine hydrochloride was added, and 720 g of water and 91.9 g of 48% aqueous sodium hydroxide solution were further added. g (1.103 mol). 79.1 g (0.562 mol) of benzoyl chloride was added dropwise while adding 46.9 g (0.562 mol) of 48% aqueous sodium hydroxide solution at around 20° C. to maintain the pH in the system at 9 to 12. Mature for 1 hour around the same temperature. Then, after adding 360 g of tetrahydrofuran and 120 g of toluene, 58.6 g (0.562 mol) of 35% aqueous hydrochloric acid solution was added dropwise at around 20°C. The pH of the reaction liquid after dripping was 2.2. After standing still, 120 g of water, 120 g of tetrahydrofuran, and 120 g of toluene were added to the organic layer obtained by liquid separation to perform extraction and washing. After standing still, the organic layer obtained by liquid separation was added ...
Embodiment 2
[0107] In a 300mL four-necked flask equipped with a thermometer, a condenser, and a stirrer, 10 g (0.046 mol) of (2R,3S)-3-phenylisoserine hydrochloride was added, followed by 50 g of water, 40 g of tetrahydrofuran, and 48% hydrogen peroxide Sodium aqueous solution 7.7g (0.092mol). 11.0 g (0.051 mol) of di-tert-butyl dicarbonate was added dropwise at around 20°C, and aged at around 40°C for 4 hours. Then, after adding 10 g of toluene, 7.2 g (0.069 mol) of 35% hydrochloric acid aqueous solution was dripped at 20 degreeC vicinity. The pH of the reaction liquid after dripping was 5.0. After standing still, the organic layer obtained by liquid separation was added to a 200 mL four-necked flask equipped with a thermometer, a condenser, a stirrer, and a Dean-Stark apparatus, and concentrated under reduced pressure while removing moisture. Furthermore, 50 g of methanol was added to the concentrate, and concentration under reduced pressure was continued until the liquid in the tank ...
Embodiment 3
[0111] In the extraction process of Example 1, it operated similarly to Example 1 except having used 1, 2- dimethoxyethane instead of tetrahydrofuran as an extraction solvent. As a result, 137.5 g (chemical purity: 99.5%, yield: 83.3%) of (2R,3S)-N-benzoyl-3-phenylisoserine methyl ester was obtained as white crystals.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap