Pyrimidine derivative and organic electroluminescence element
一种嘧啶衍生物、致发光的技术,应用在电固体器件、电气元件、发光材料等方向,能够解决缺乏阻挡、缺乏稳定性空穴阻挡层、缺乏稳定性等问题,达到改善耐电流性、优异空穴阻挡能力、改善最大亮度的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0194]
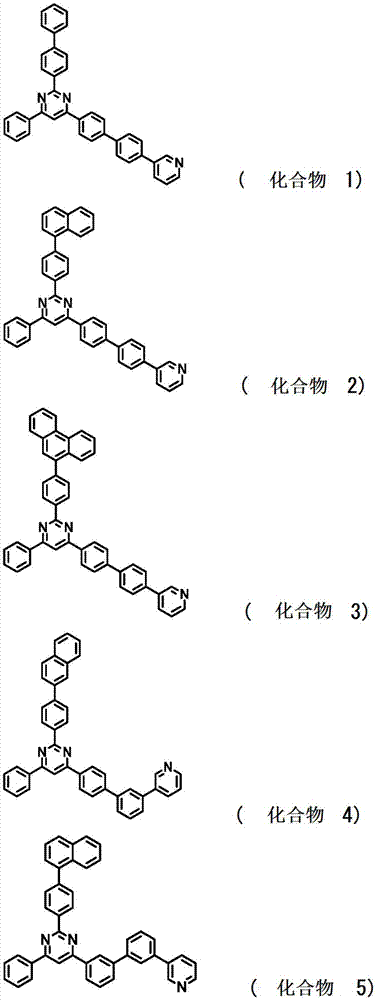
[0195] Synthesis of 2-(biphenyl-4-yl)-4-phenyl-6-{4’-(pyridin-3-yl)biphenyl-4-yl}pyrimidine;
[0196]
[0197] It was added to a reaction vessel purged with nitrogen, heated and stirred at 80°C for 12 hours to prepare a reaction solution. The reaction solution was cooled to room temperature, and the organic layer was collected by a liquid separation operation. Then, the solution was concentrated under reduced pressure to obtain a crude product, followed by purification by column chromatography (carrier: silica gel, eluent: ethyl acetate / heptane), and then, by recrystallization by using a mixed solvent of tetrahydrofuran / acetone. Obtain 3.0g of 2-(biphenyl-4-yl)-4-phenyl-6-{4'-(pyridin-3-yl)biphenyl-4-yl)pyrimidine (compound 1) as a white powder Rate, 30%).
[0198]
[0199] The structure of the obtained white powder was identified by NMR. Figure 24 show 1 The result of H-NMR measurement. by 1 H-NMR(CDCl 3 ) The following 27 hydrogen signals were detected.
[0200] δ(ppm...
Embodiment 2
[0206]
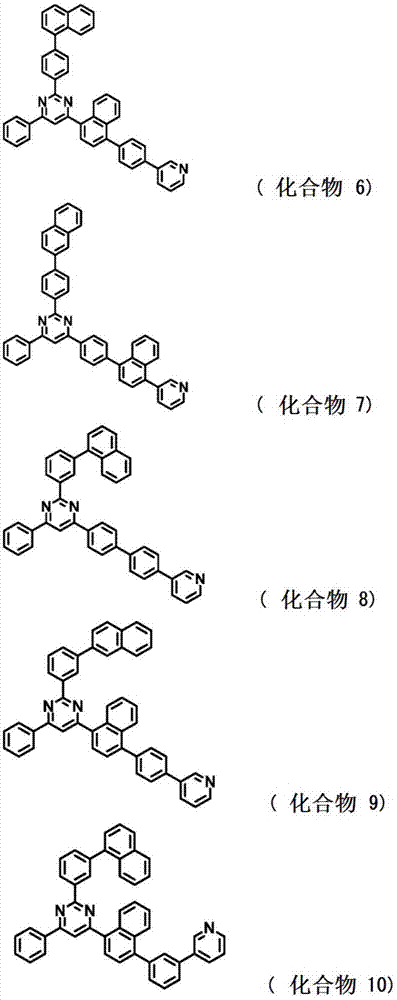
[0207] Synthesis of 2-{4-(naphthalene-1-yl)phenyl}-4-phenyl-6-{4’-(pyridin-3-yl)biphenyl-4-yl}pyrimidine;
[0208] In addition to using
[0209] {4-(Naphthalene-1-yl)phenyl}boronic acid
[0210] Use instead
[0211] Other than 4-biphenylboronic acid,
[0212] The reaction was carried out under the same conditions as in Example 1.
[0213] As a result, 1.6 g of 2-{4-(naphthalen-1-yl)phenyl}-4-phenyl-6-{4'-(pyridin-3-yl)biphenyl-4-yl}pyrimidine (compound 2) White powder (yield, 15%).
[0214]
[0215] The structure of the obtained white powder was identified by NMR. Figure 25 show 1 The result of H-NMR measurement. by 1 H-NMR(CDCl 3 ) The following 29 hydrogen signals were detected.
[0216] δ(ppm)=9.00-8.81(3H)
[0217] 8.65(1H)
[0218] 8.51-8.28(4H)
[0219] 8.11-7.32(21H)
Embodiment 3
[0220]
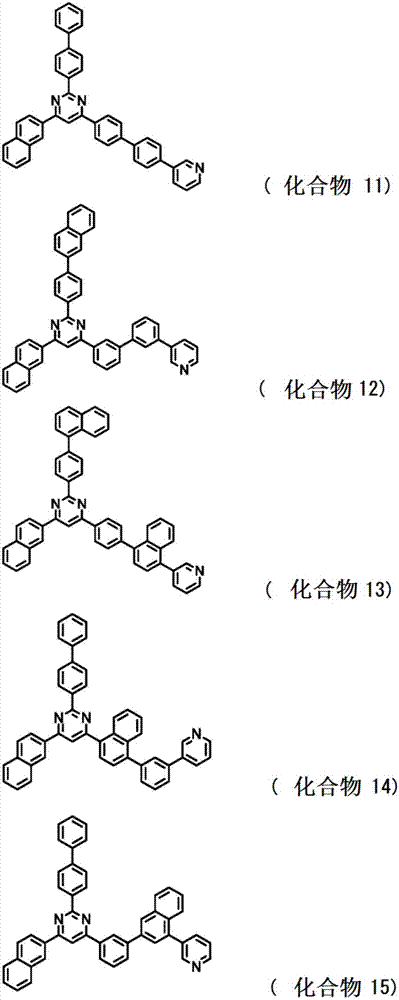
[0221] Synthesis of 2,4-bis(phenanthrene-9-yl)-6-{4’-(pyridin-3-yl)biphenyl-4-yl}pyrimidine;
[0222] In addition to using
[0223] 2-chloro-4-(phenanthrene-9-yl)-6-{4’-(pyridin-3-yl)biphenyl-4-yl}pyrimidine
[0224] Use instead
[0225] 2-chloro-4-phenyl-6-{4’-(pyridin-3-yl)biphenyl-4-yl}pyrimidine,
[0226] and use
[0227] Phenanthrene-9-boric acid
[0228] Use instead
[0229] Other than 4-biphenylboronic acid,
[0230] The reaction was carried out under the same conditions as in Example 1.
[0231] As a result, 1.2 g of white powder of 2,4-bis(phenanthrene-9-yl)-6-{4'-(pyridin-3-yl)-biphenyl-4-yl}pyrimidine (compound 29) was obtained (yield Rate, 14%).
[0232]
[0233] The structure of the obtained white powder was identified by NMR. Figure 26 show 1 The result of H-NMR measurement. by 1 H-NMR(CDCl 3 ) The following 31 hydrogen signals were detected.
[0234] δ(ppm)=9.05-8.35(14H)
[0235] 8.25-7.52(15H)
[0236] 7.45-7.35(2H)
PUM
| Property | Measurement | Unit |
|---|---|---|
| electron work function | aaaaa | aaaaa |
| electron work function | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com