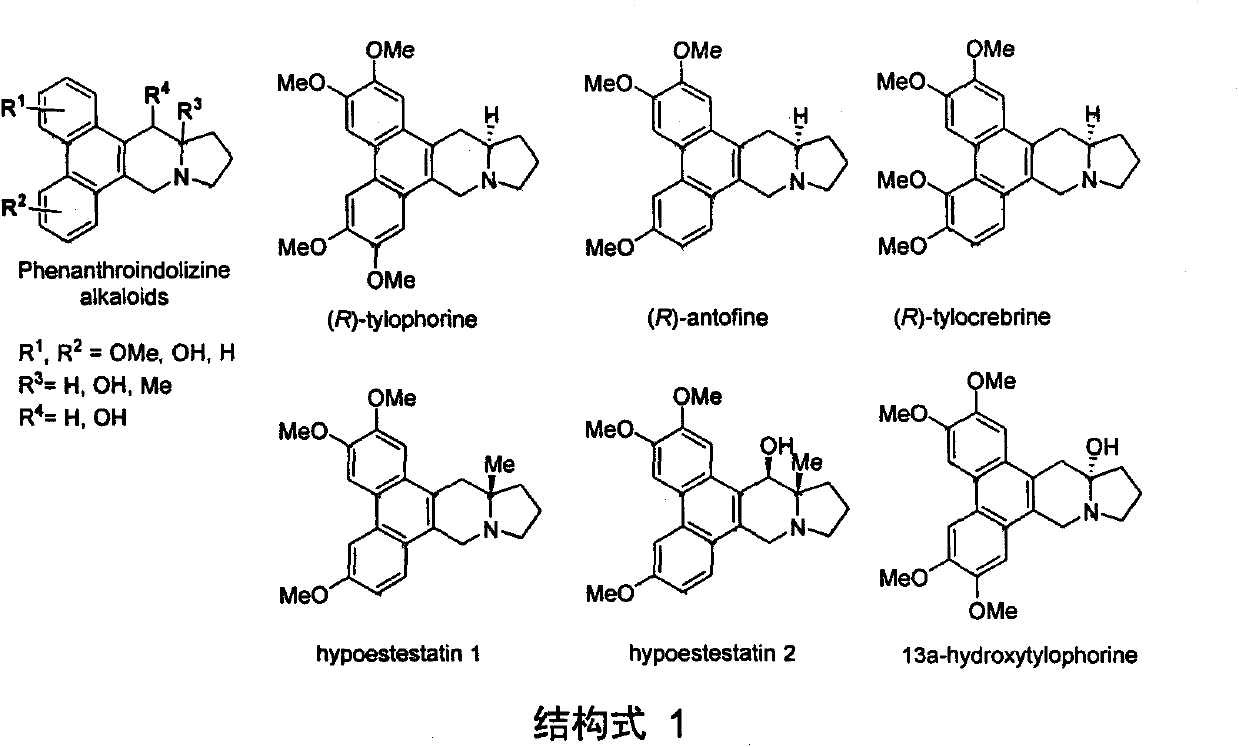
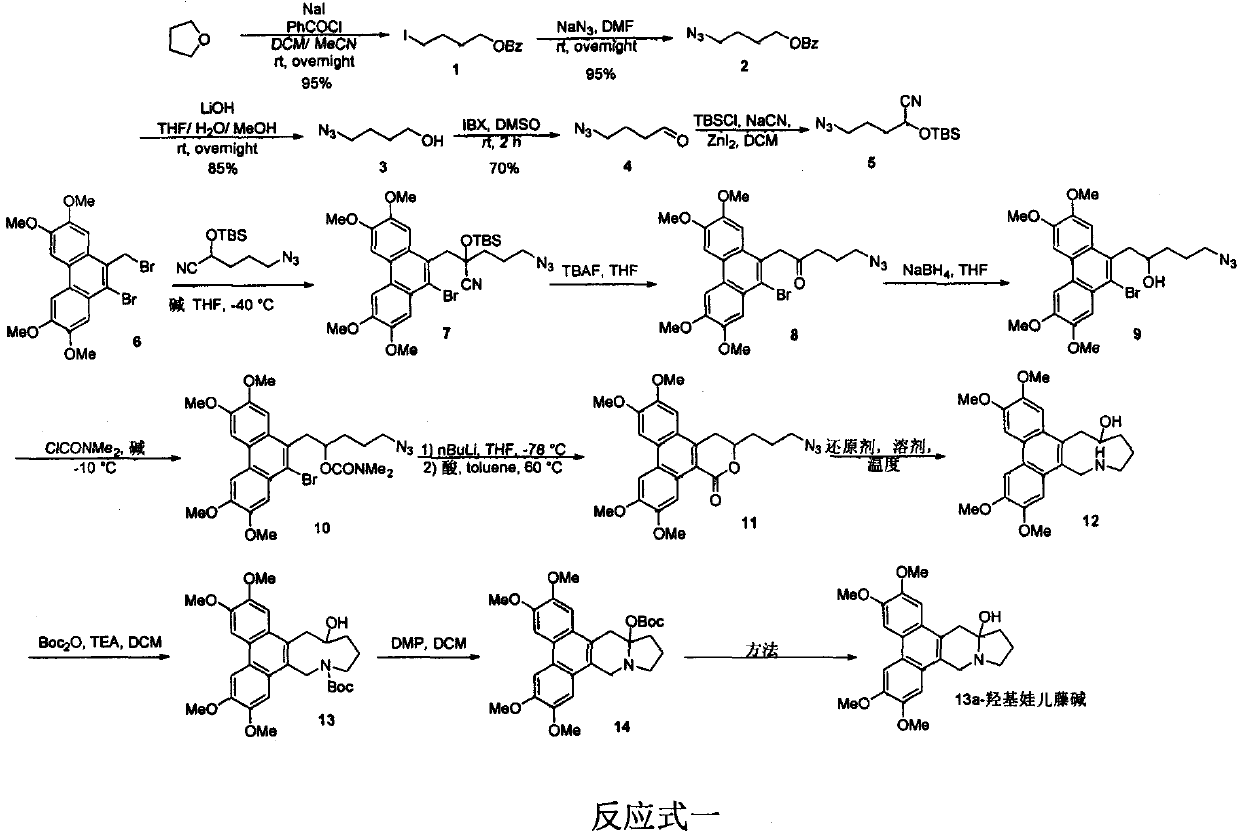
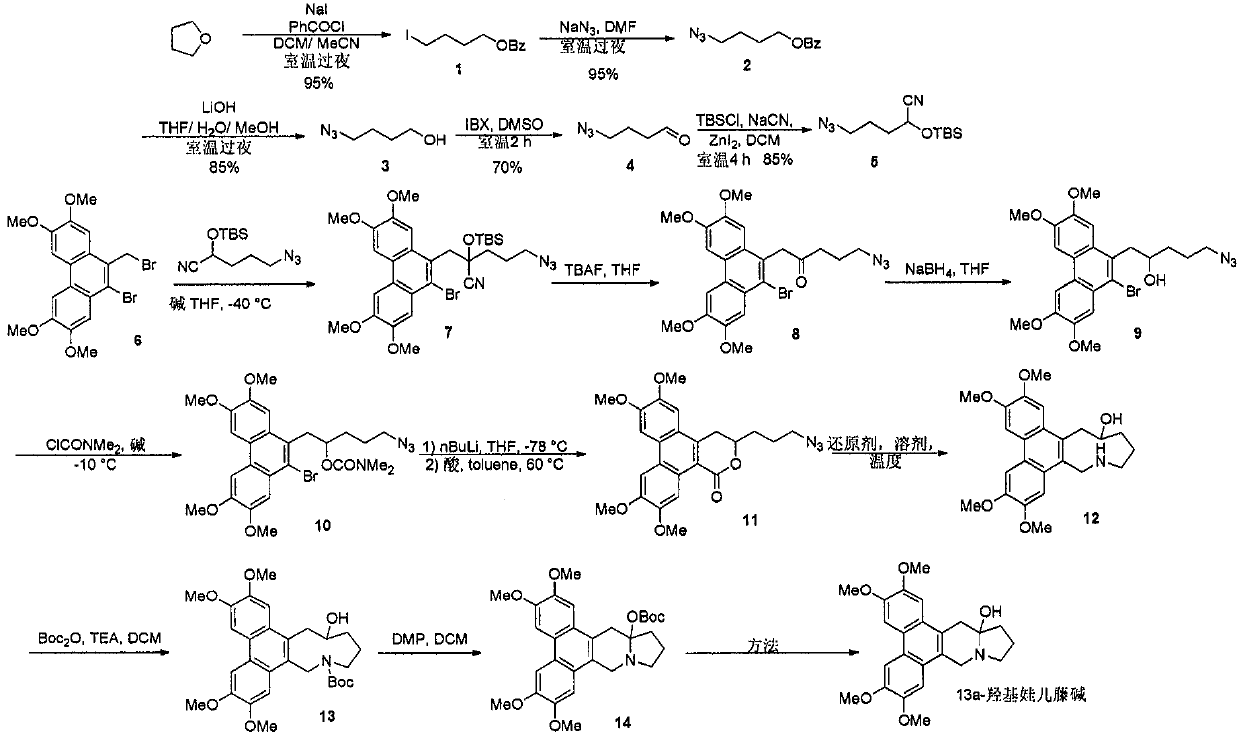
A new method for the total synthesis of 13a-hydroxysiliphenine
A technology of total synthesis of hydroxysilicate, applied in organic chemistry, bulk chemical production, etc., can solve the problems of blank synthesis of 13a-hydroxysilicate, less separation content, no synthesis research, etc., and achieve novel structure , mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0021] Synthesis of Intermediate 1: Add sodium iodide (27.9g, 0.186mol), 15mL tetrahydrofuran, and 30mL acetonitrile into a 500mL single-necked bottle, add benzoyl chloride (17.3ml, 0.15mol) dropwise in an ice bath, and complete the addition in about half an hour , react overnight at room temperature. The reaction solvent was distilled off under reduced pressure, the residue was diluted with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and precipitated to obtain 43.2 g of a colorless liquid with a yield of 95%. 1 H NMR (400MHz, CDCl 3 ): δ8.01(m, 2H), 7.53(t, J=7.3Hz, 1H), 7.43(t, J=7.8Hz, 2H), 4.34(t, J=6.4Hz, 2H), 3.24(t , J=6.8Hz, 2H), 1.88-1.99(m, 4H).
[0022] Synthesis of Intermediate 2: Add 1 (21.6g, 0.071mol), 100mL DMF, and sodium azide (6.92g, 0.107mol) to a 500mL single-necked bottle, and react at room temperature for 6h. The reaction solution was diluted with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com