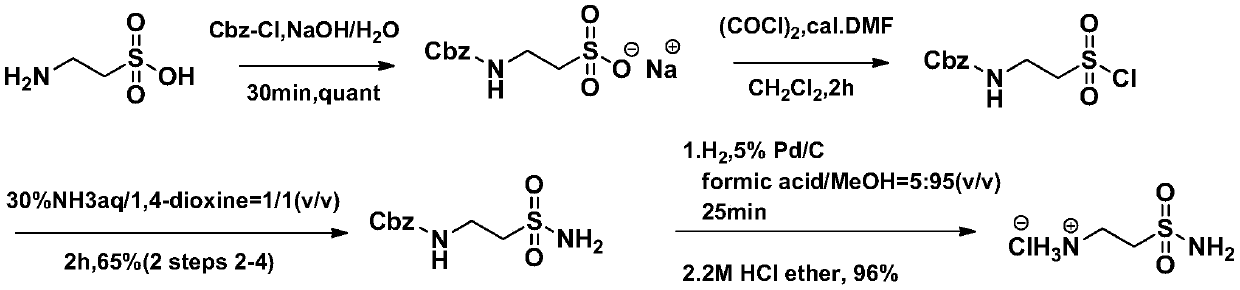
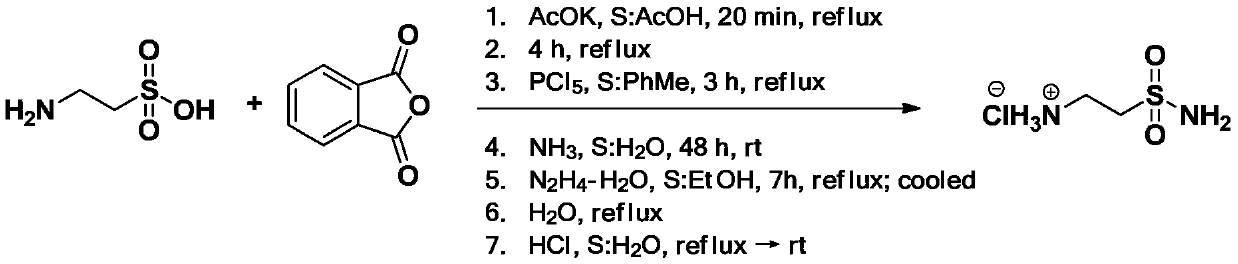
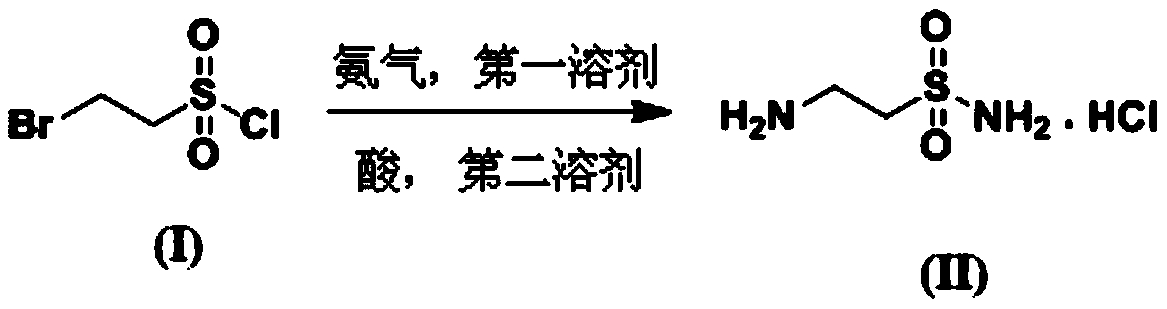
A kind of synthetic method of taurine amide hydrochloride (2-aminoethylsulfonamide hydrochloride)
A technology of aminoethylsulfonamide and taurine hydrochloride, which is applied in the field of synthesis of taurine hydrochloride, can solve the problems of difficult removal of water, time-consuming post-processing, and long process time, achieving less side reactions, Easy post-processing and simple reaction treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] 1.1 Preparation of compound (2-aminoethylsulfonamide hydrochloride / tauronamide hydrochloride)
[0034] Under the ice-water bath, add 10ml redistilled tetrahydrofuran into the 50ml three-necked flask, stir, the system is evacuated to replace the ammonia gas, so that it is in an ammonia gas environment of 1.0 atmosphere, and 1.5g compound (2-bromoethylsulfonyl chloride) is dissolved in Add 10ml of dry tetrahydrofuran into the system dropwise through a constant pressure dropping funnel within 10 minutes, and it can be observed that a white solid is formed in the system. Under an ice-water bath, continue to stir and react for 2 hours. The solution is clear and white lumps are formed at the bottom.
[0035] At 30°C, evaporate the solvent to dryness, add saturated sodium bicarbonate solution to adjust the pH between 7 and 8, add dichloromethane for multiple extractions, dry with anhydrous sodium sulfate, and evaporate to dryness, then beat with ethyl acetate, pump Filter to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com