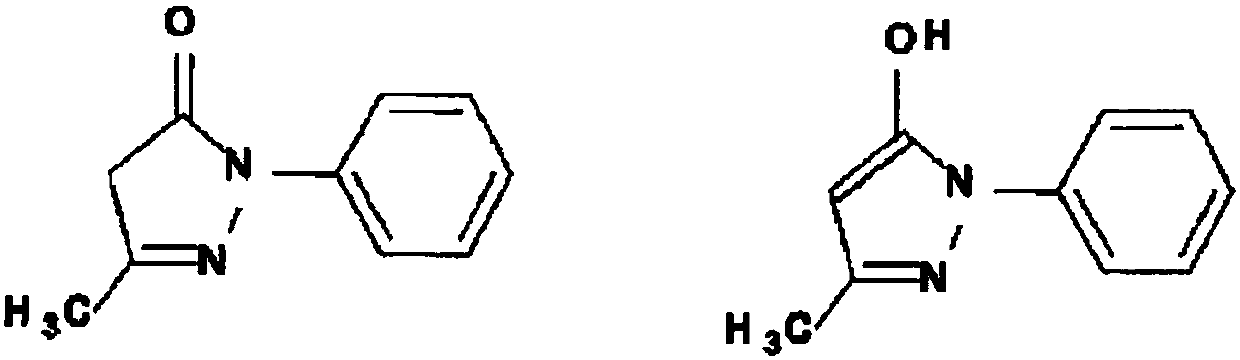
Medicinal agent for treating amyotrophic lateral sclerosis or preventing progression of phase of amyotrophic lateral sclerosis
A technology for lateral sclerosis and muscular atrophy, applied in muscular system diseases, neuromuscular system diseases, medical preparations containing active ingredients, etc. Questions such as frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0142] (experiment method)
[0143] 199 ALS patients meeting the following selection criteria were divided into an actual drug administration group of 100 and a placebo administration group of 99.
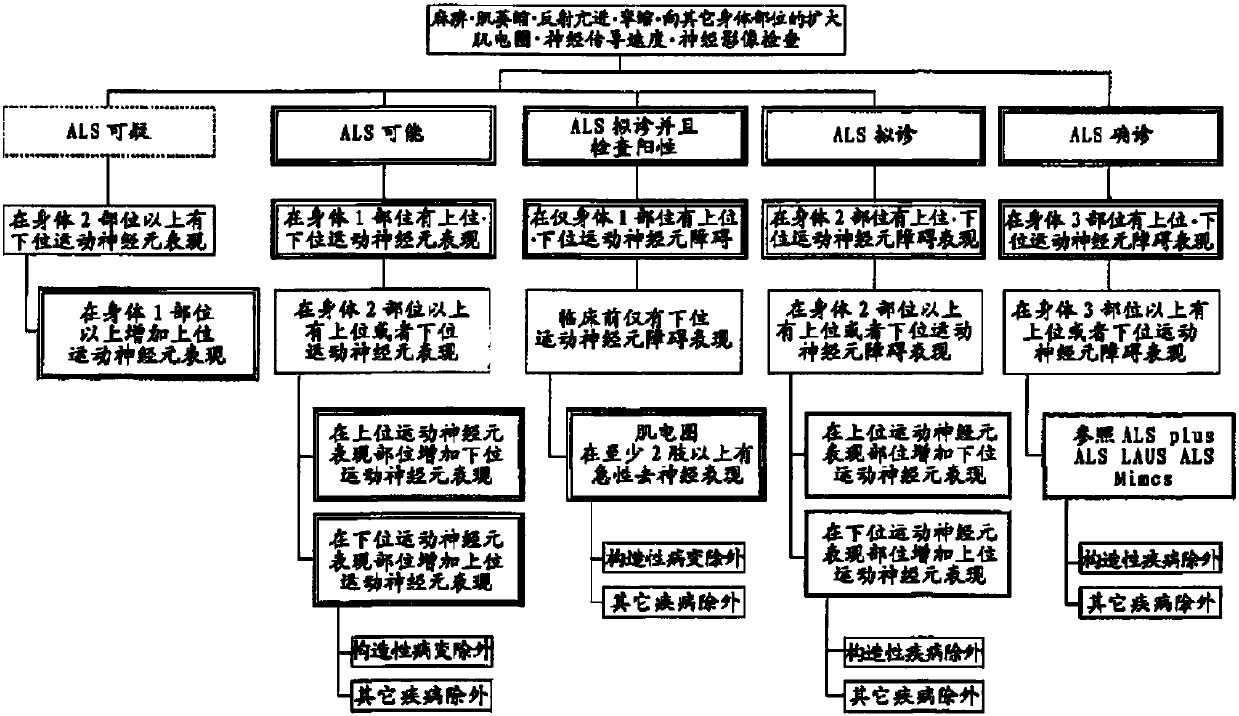
[0144] 1) According to EL Escorial's revised Airlie House diagnostic criteria, it is suitable for any one of "ALS confirmed", "ALS probable", "ALS probable and positive" or "ALS probable"
[0145] 2) Can eat, excrete, and move without assistance in daily life
[0146] 3) Within 3 years after the onset of symptoms of ALS
[0147] 4) Age is greater than or equal to 20 years old and less than or equal to 75 years old
[0148] 5) The amount of change in the ALSFRS-R score 12 weeks before the start of administration is -1 to -4 points
[0149] 6) No dyspnea caused by decreased respiratory function
[0150] 7) There are no complications such as Parkinson's disease, schizophrenia, dementia, etc. that may affect the evaluation of drug efficacy
[0151] In the actual drug administratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com