Recovery method for cathode material of waste lithium iron phosphate battery
A lithium iron phosphate battery and cathode material technology, applied in battery recycling, waste collector recycling, recycling technology, etc., can solve problems such as complex process routes, increased costs, and poor performance of lithium-ion batteries, and achieve shortened process Process, cost saving, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
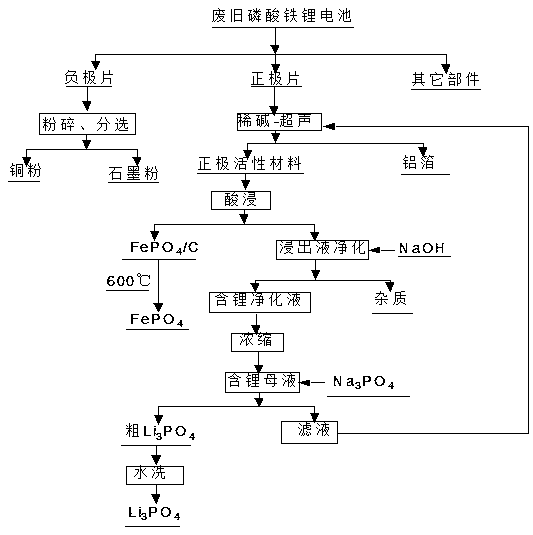
[0023] A method for recycling the positive electrode material of a waste lithium iron phosphate battery, comprising the following steps:
[0024] 1) Discharge, disassemble, and separate the waste lithium iron phosphate battery to obtain the battery core and shell. The battery core is disassembled and separated to obtain the positive electrode sheet containing the lithium iron phosphate positive electrode active material, which is placed in a 0.4mol / l NaOH solution Ultrasonic machine treatment for 10 minutes to make lithium iron phosphate positive electrode active material and aluminum foil;
[0025] 2) Take 100g of the lithium iron phosphate cathode active material obtained in step 2) to react with sulfuric acid + hydrogen peroxide, lithium and acid H + The molar ratio of lithium to H is 1:0.98 2 o 2 The molar ratio of the hydrogen peroxide is 1:0.5, and the hydrogen peroxide can oxidize ferrous iron to ferric iron while promoting the reaction speed of the experiment. Other...
Embodiment 2
[0029] A method for recycling the positive electrode material of a waste lithium iron phosphate battery, comprising the following steps:
[0030] 1) Discharge, disassemble, and separate the waste lithium iron phosphate battery to obtain the battery cell and shell. The battery cell is disassembled and separated to obtain the positive electrode sheet containing the lithium iron phosphate positive electrode active material, which is placed in a 0.35mol / l NaOH solution Ultrasonic machine treatment for 10 minutes to make lithium iron phosphate positive electrode active material and aluminum foil;
[0031] 2) Take 100g of the lithium iron phosphate cathode active material obtained in step 2) to react with hydrochloric acid + hydrogen peroxide, lithium and acid H + The molar ratio is 1:1, Li and H 2 o 2 The molar ratio of the hydrogen peroxide is 1:0.52, and the hydrogen peroxide can oxidize ferrous iron to ferric iron while promoting the reaction speed of the experiment. Other re...
Embodiment 3
[0035] A method for recycling the positive electrode material of a waste lithium iron phosphate battery, comprising the following steps:
[0036] 1) Discharge, disassemble and separate the waste lithium iron phosphate battery to obtain the battery cell and shell. The battery cell is disassembled and separated to obtain the positive electrode sheet containing the lithium iron phosphate positive electrode active material, and placed in an ultrasonic machine containing 0.45mol / l NaOH solution for 15 minutes to make the lithium iron phosphate positive electrode active material and aluminum foil;
[0037] 2) Take 100g of the lithium iron phosphate cathode active material obtained in step 2) to react with bromic acid + hydrogen peroxide, lithium and acid H + The molar ratio of lithium and H is 1:1.02 2 o 2 The molar ratio of the hydrogen peroxide is 1:1.55, and the hydrogen peroxide can oxidize ferric iron to ferric iron while promoting the reaction speed of the experiment. Other...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com