Compound containing pyridine and thiophene as well as preparation method and application thereof
A compound, thiophene carboxylic acid technology, applied in the field of medicine, can solve problems such as insufficient cure of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
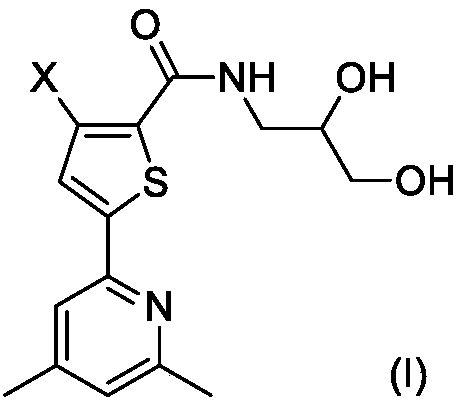
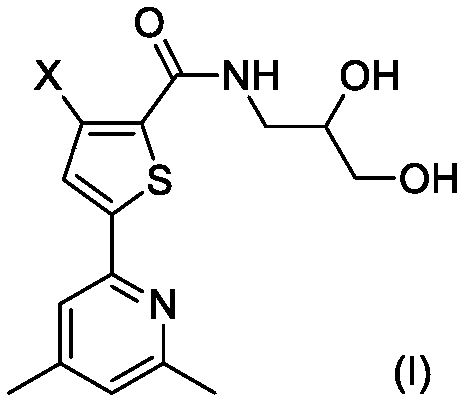
[0025] The synthesis of embodiment 1 compound I-1
[0026]
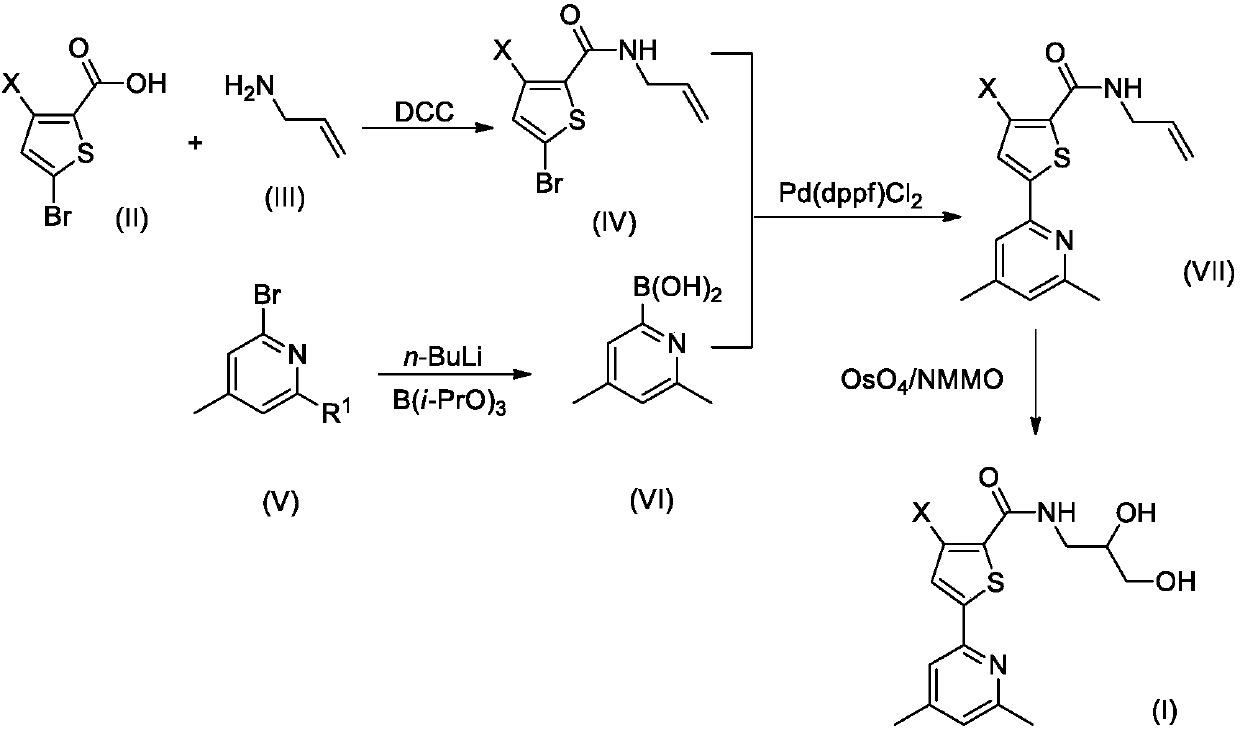
[0027] Step 1. Synthesis of compound IV-1
[0028] 2.07g (10mmol) of compound II-1 and 0.57g (10mmol) of compound III were dissolved in 20mL of dry THF, stirred at room temperature, and 2.48g (12mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added and 0.1 g of 4-dimethylaminopyridine (DMAP), after the addition was complete, the reaction mixture was stirred overnight at room temperature, and TLC tracking found that the reaction was complete.
[0029] The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to ob...
Embodiment 2
[0039] The synthesis of embodiment 2 compound 1-2
[0040]
[0041] Step 1. Synthesis of compound IV-2
[0042] 2.25g (10mmol) of compound II-2 and 0.57g (10mmol) of compound III were dissolved in 20mL of dry THF, stirred at room temperature, and 2.48g (12mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added and 0.1 g of 4-dimethylaminopyridine (DMAP), after the addition was complete, the reaction mixture was stirred overnight at room temperature, and TLC tracking found that the reaction was complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain com...
Embodiment 3
[0049] The synthesis of embodiment 3 compound 1-3
[0050]
[0051] Step 1. Synthesis of compound IV-3
[0052] 2.41g (10mmol) of compound II-3 and 0.57g (10mmol) of compound III were dissolved in 20mL of dry THF, stirred at room temperature, and 2.48g (12mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added and 0.1 g of 4-dimethylaminopyridine (DMAP), after the addition was complete, the reaction mixture was stirred overnight at room temperature, and TLC tracking found that the reaction was complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com