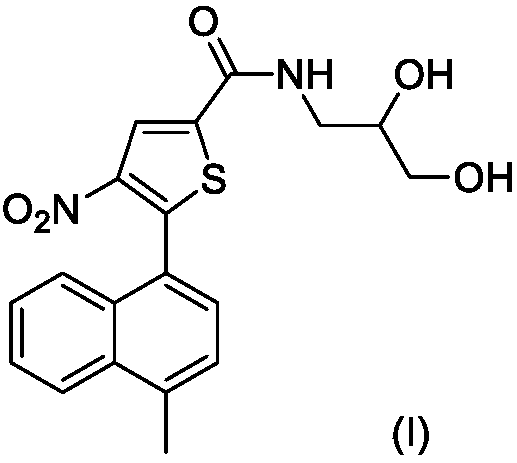
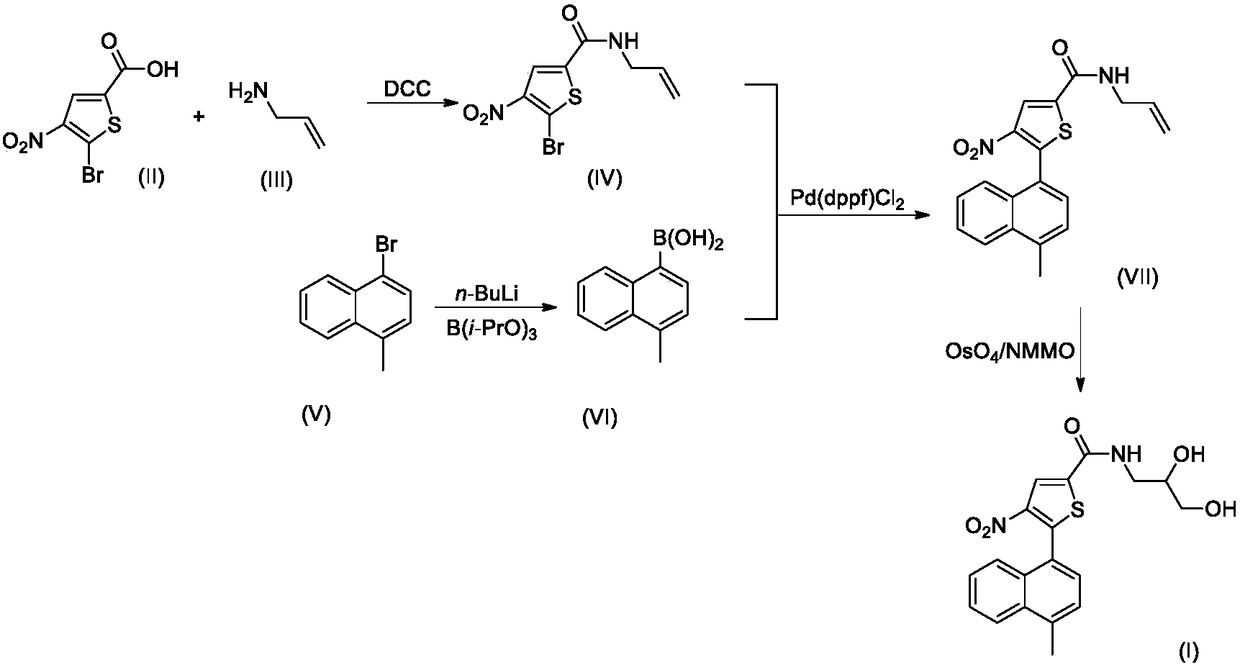
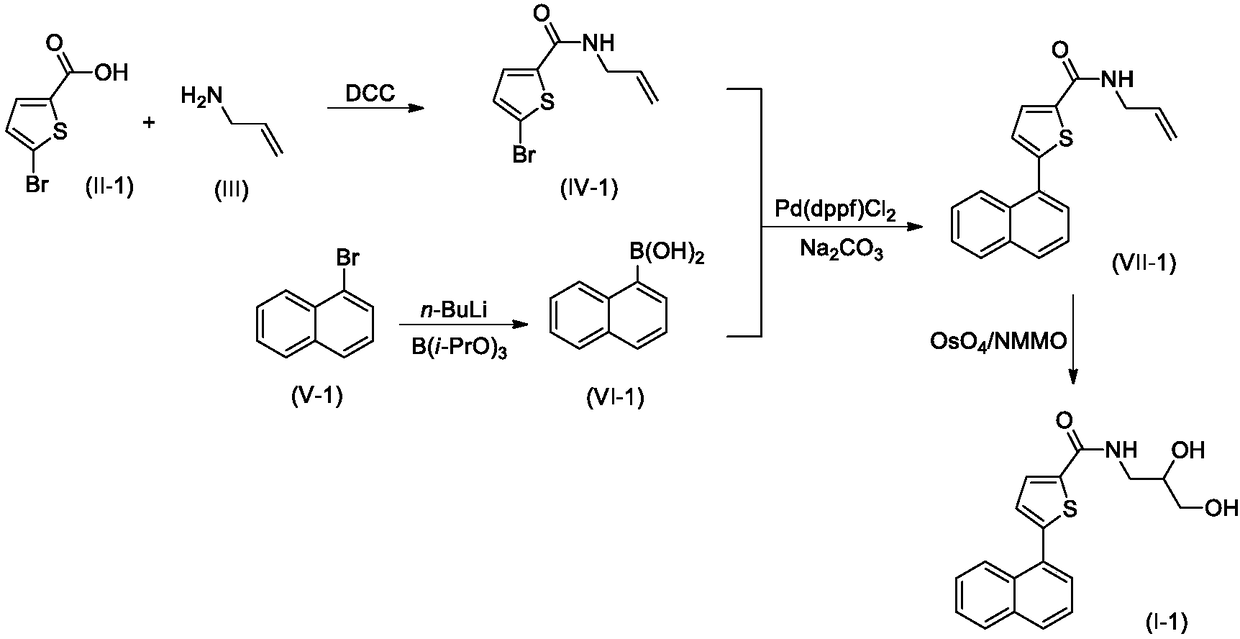
ROCK inhibitor comprising methylnaphthalene and propanediol thiophene amide structure
A technology of allylamine and thiophene carboxylic acid, applied in the field of medicine, can solve problems such as not enough to cure diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The synthesis of embodiment 1 compound I-1
[0028]
[0029] Step 1. Synthesis of compound IV-1
[0030] 2.07g (10mmol) of compound II-1 and 0.57g (10mmol) of compound III were dissolved in 20mL of dry THF, stirred at room temperature, and 2.48g (12mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added and 0.1 g of 4-dimethylaminopyridine (DMAP), after the addition was complete, the reaction mixture was stirred overnight at room temperature, and TLC tracking found that the reaction was complete.
[0031] The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to ob...
Embodiment 2
[0041] The synthesis of embodiment 2 compound 1-2
[0042]
[0043] Step 1. Synthesis of compound IV-2
[0044] 2.52g (10mmol) of compound II-1 and 0.57g (10mmol) of compound III were dissolved in 20mL of dry THF, stirred at room temperature, and 2.48g (12mmol) of N,N'-dicyclohexylcarbodiimide (DCC) was added and 0.1 g of 4-dimethylaminopyridine (DMAP), after the addition was complete, the reaction mixture was stirred overnight at room temperature, and TLC tracking found that the reaction was complete. The reaction mixture was carefully poured into 200mL ice water, stirred, and washed with 50mL×3CH 2 Cl 2 After extraction, the extract phases were combined, washed successively with 100 mL of 1% dilute hydrochloric acid and 100 mL of 5% brine, and dried over anhydrous sodium sulfate. The desiccant was removed by suction filtration, the filtrate was evaporated to dryness on a rotary evaporator, and the residue was purified by silica gel column chromatography to obtain com...
Embodiment 3
[0051] Example 3 compound in vitro inhibition of ROCK analysis
[0052]Inhibition experiments were performed by the fluorescence polarization (FP) method using a commercially available ROCK IMAP kit from Molecular Devices (Product No. R8093) and essentially according to the protocol provided by the manufacturer. The S6 ribosomal protein-derived substrate used was (Fl)-AKRRRLSSLRA, also from Molecular Devices (product number R7184). Enzyme mix of ROCKα / ROCKII was obtained from Upstate Biotechnology (product number 14-451). Briefly, all compounds were screened in 384-well plates to determine enzyme inhibition at concentrations ranging from 100 μM to 0.1 nM using serial 3-fold dilutions. Y-27632 (commercially available from Tocris Corporation) was used as a reference (0.4 μM) in order to perform the experiments. 1 μL of compound solution (each concentration) to be tested in DMSO was placed in 2 μL of enzyme solution with 10 mM Tris-HCl, 10 mM MgCl2, 0.1% BSA, 0.05% NaN3, pH 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com