CD22 targeted chimeric antigen receptor and use thereof
A receptor and single-chain antibody technology, applied in the field of CD22-targeted chimeric antigen receptors, can solve problems beyond the treatment requirements, T cell attack, and high amplification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
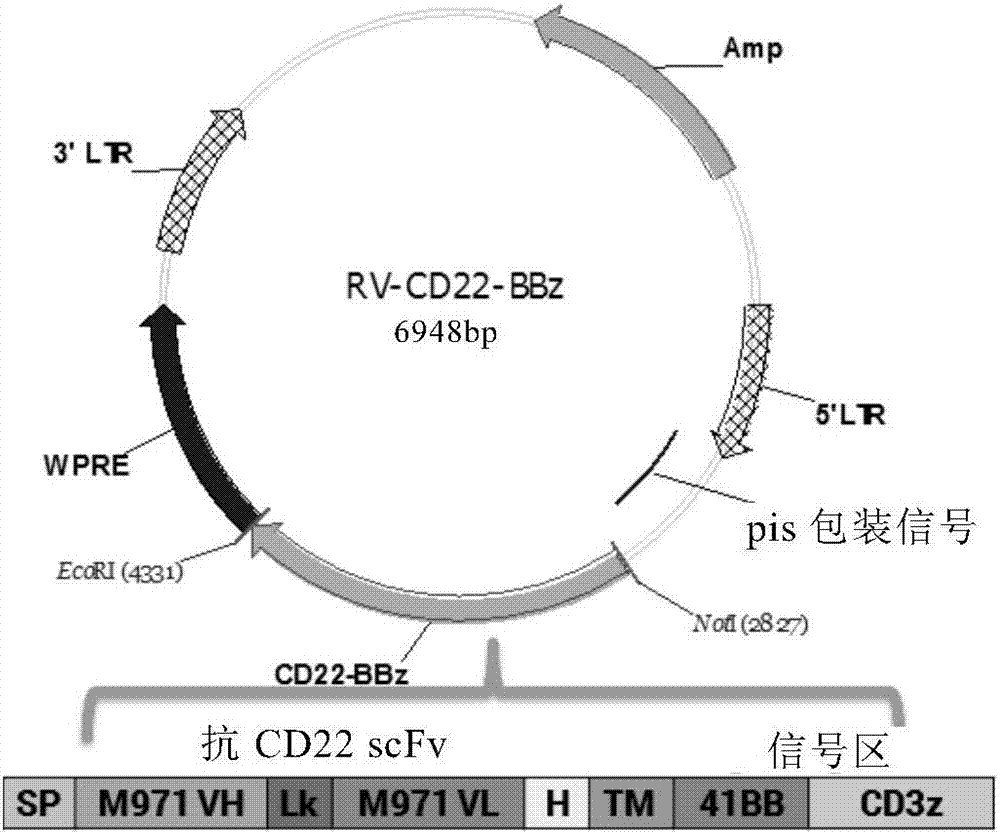
[0088] Example 1: Determination of CD22-scFv-CD8α-CD28-41BB-CD3ζ gene sequence
[0089] The gene sequence information of human CD8α hinge region, human CD8α transmembrane region, 41BB intracellular region and human CD3ζ intracellular region was searched from the NCBI website database. The anti-CD22 single-chain antibody clone number is m971. These sequences are available on the website http: / / sg Codon optimization is performed on .idtdna.com / site to ensure that it is more suitable for expression in human cells without changing the encoded amino acid sequence.
[0090] Using overlapping PCR, the above sequences were sequentially connected according to anti-CD22 scFv, human CD8α hinge region gene, human CD8α transmembrane region gene, 41BB intracellular region gene, and human CD3ζ intracellular region gene sequence, and different enzyme cutting sites were introduced at the junction of each sequence Click to form the complete CD22CAR gene sequence information.
[0091] The nucle...
Embodiment 2
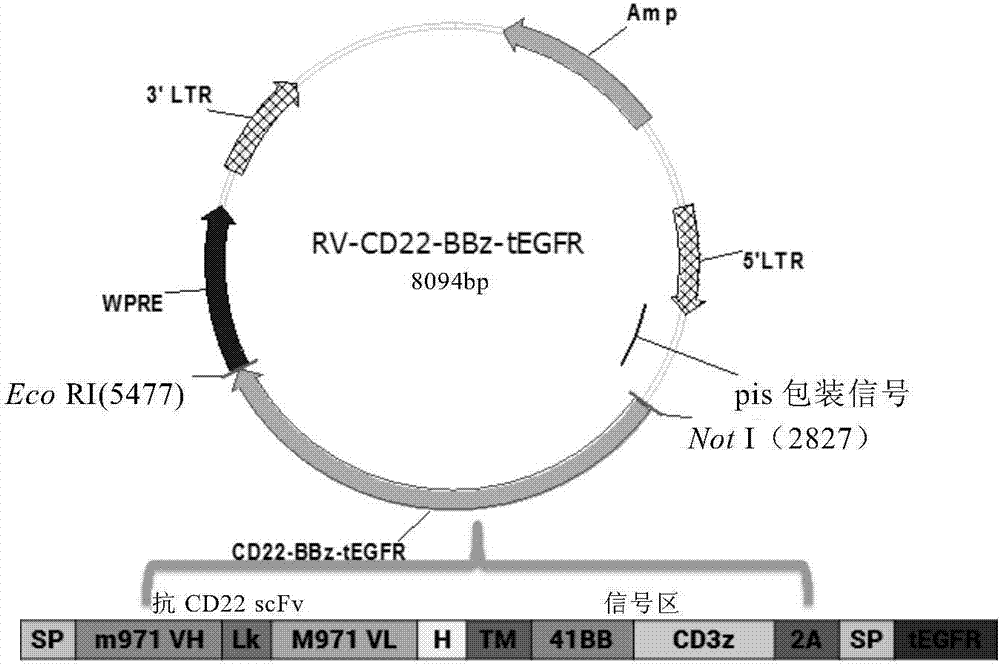
[0097] Example 2: Determination of CD22CAR-GMCSFR leader sequence-tEGFR gene sequence
[0098] The gene sequence information of the extracellular region of human EGFR was searched from the NCBI website database, and the sequence was codon-optimized on the website http: / / sg.idtdna.com / site to ensure that it is more suitable for human cell expression under the condition that the encoded amino acid sequence remains unchanged .
[0099] Using overlapping PCR, the above sequences were sequentially connected according to the CD22CAR, GMCSFR leader sequence, and tEGFR in Example 1, and different restriction sites were introduced at the junction of each sequence to form a complete CD22CAR-GMCSFR leader sequence-tEGFR gene sequence information.
[0100] The nucleotide sequence of the CAR molecule was double-digested with NotI (NEB) and EcoRI (NEB), connected and inserted into the NotI-EcoRI site of retrovirus MSCV (Addgene) by T4 ligase (NEB), and transformed into competent large intes...
Embodiment 3
[0106] Example 3: Retroviral packaging
[0107] 1. Day 1: 293T cells should be less than 20 passages, not overgrown. Take 0.6×10 6 Cells / ml were plated, and 10ml of DMEM medium was added to a 10cm dish, the cells were thoroughly mixed, and cultured overnight at 37°C.
[0108] 2. Day 2: 293T cell confluency reaches about 90% for transfection (usually about 14-18 hours after plating); prepare plasmid complexes, the amount of various plasmids is MSCV backbone 12.5ug, Gag-pol 10ug, VSVg 6.25 Ug, CaCl 2 250ul,H 2 O 1ml, the total volume is 1.25ml; add HBS equal to the volume of the plasmid complex in another tube, and vortex for 20 seconds while adding the plasmid complex. Gently add the mixture to the 293T dish along the side, incubate at 37°C for 4 hours, remove the medium, wash with PBS, and add pre-warmed fresh medium again.
[0109] 3. Day 4: 48 hours after transfection, collect the supernatant and filter it with a 0.45um filter, store in -80°C, and continue to add prehea...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap