Method for rapidly and quantitatively evaluating quality stability of traditional Chinese medicine injection
A quantitative evaluation and injection technology, applied in the measurement of color/spectral properties, etc., can solve the problems that cannot be confirmed theoretically, and achieve the effect of fast quantitative evaluation of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
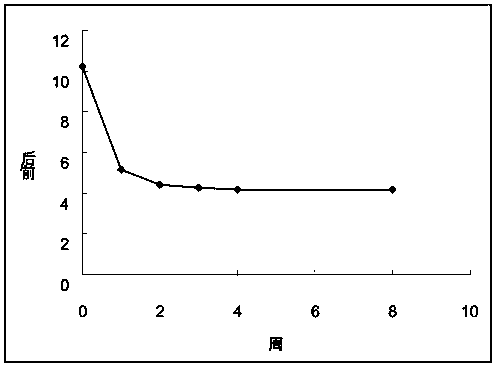
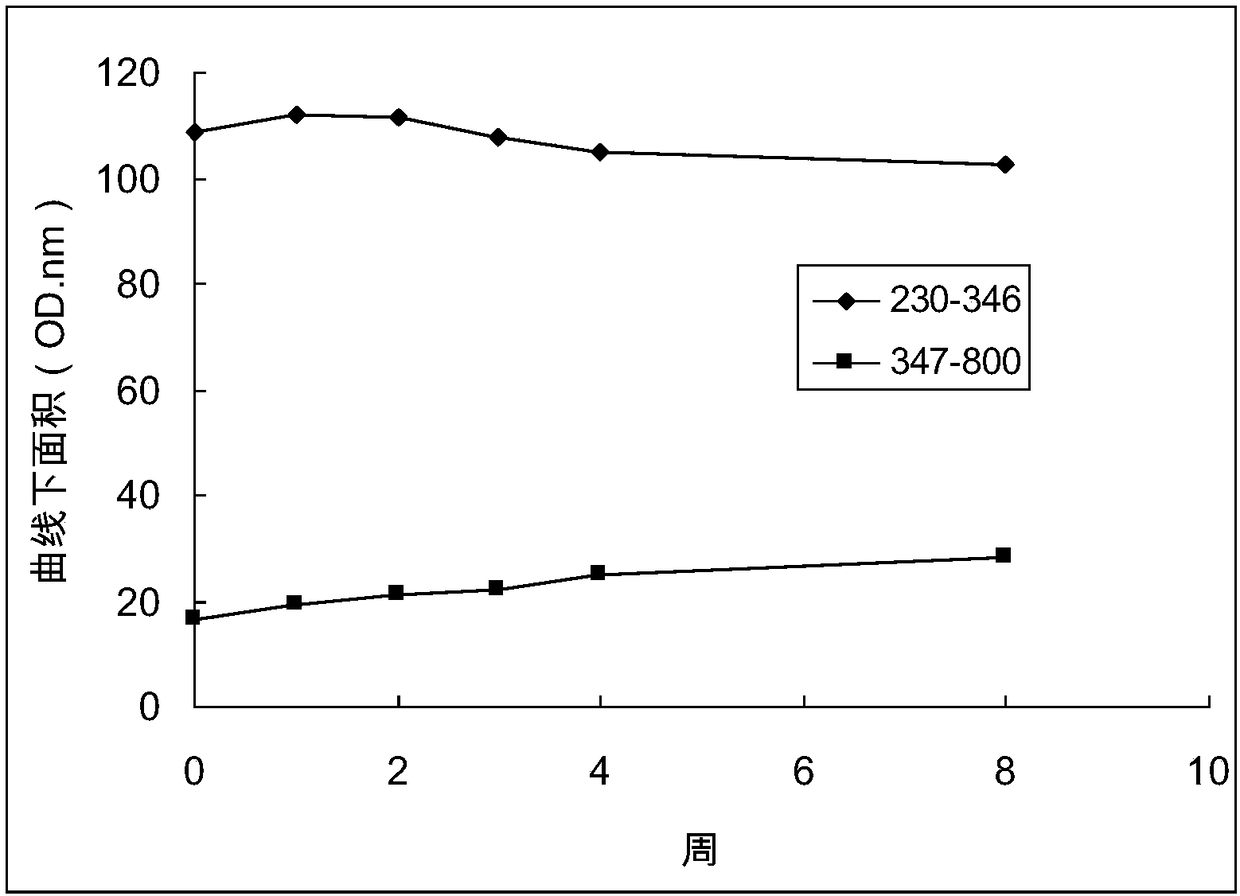
[0056] Example 1 Changes in the isosbestic point and area under the curve of Qingkailing injection.
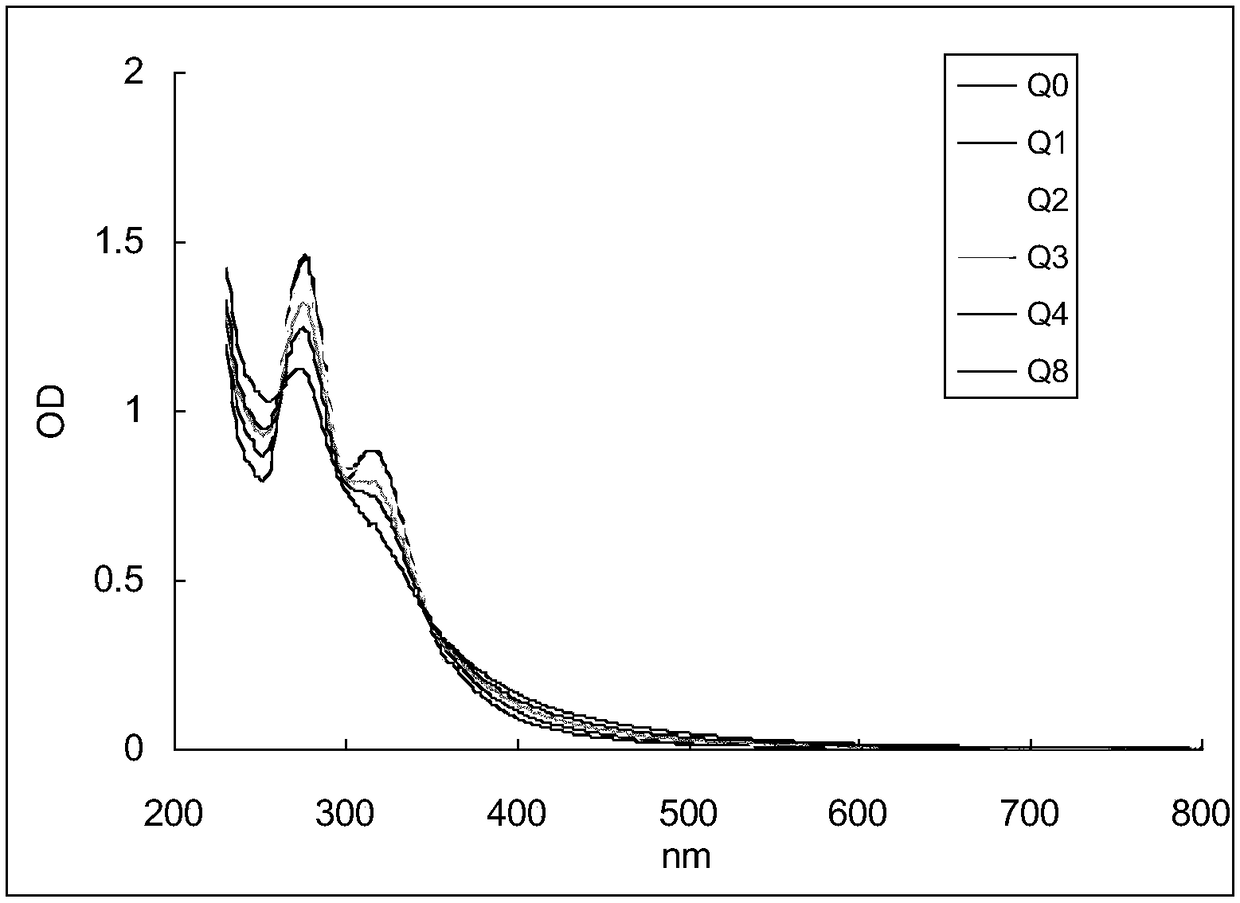
[0057] Take 10ml of Qingkailing injection (batch number 1003272) purchased in the market, take a sample of 1.0ml for later use (recorded as standard sample Q0), add the remaining injection into a 15ml capped centrifuge tube, close the cap tightly and place it in a 60°C water bath for constant temperature . After 1, 2, 3, 4, and 8 weeks, 1.0 ml samples were taken at fixed time points (the samples were respectively marked as Q1, Q2, Q3, Q4, and Q8). After the sample is taken, it is sealed and stored at -20°C. After the sample is taken, the absorption spectrum detection is performed, and the fingerprint detection is performed in parallel to verify from the side (reference literature: Wang Zhihong, Zhao Xuyuan, Yao Jincheng. Qingkailing injection fingerprint HPLC study of the spectrum. Chinese Journal of Traditional Chinese Medicine 2008; 26:868-70).
[0058] Absorption spectrum...
Embodiment 2
[0067] Example 2 Changes in isosbestic point and area under the curve of Shuanghuanglian injection.
[0068]Take 10ml of Shuanghuanglian injection (batch number 20100324) purchased in the market, sample 1.0ml for later use (denoted as standard sample S0), add the remaining injection into a 15ml capped centrifuge tube, close the cap tightly and place it in a 60°C water bath for constant temperature. After 1, 2, 3, 4, and 8 weeks, 1.0 ml samples were taken at fixed time points (the samples were respectively marked as S1, S2, S3, S4, and S8). After the sample is taken, it is sealed and stored at -20°C. After the sample is taken, the absorption spectrum is detected, and the fingerprint is detected in parallel to verify from the side (reference literature: Li Fang, Jiang Wenhong, Liu Lijuan, Zhong Zhaoqing. Injection Establishment of fingerprints of Shuanghuanglian (lyophilized) and its application in quality control. Chinese Patent Medicine 2007;29:1196-8).
[0069] Absorption sp...
Embodiment 3
[0080] Example 3 Changes in the isosbestic point and area under the curve of Danshen injection.
[0081] Take 10ml of Danshen injection (batch number 1005104) purchased in the market, sample 1.0ml for later use (denoted as standard sample D0), add the remaining injection into a 15ml capped centrifuge tube, close the cap tightly and place it in a water bath at 60°C for constant temperature. After 1, 2, 3, 4, and 8 weeks, 1.0 ml samples were taken at fixed time points (the samples were respectively marked as D1, D2, D3, D4, and D8). After the sample is taken, it is sealed and stored at -20°C. After the sample is taken, the absorption spectrum detection is carried out, and the fingerprint detection is performed in parallel to verify from the side (reference literature: Xu Man, Liu Aihua, Cui Yajun, Zhang Jinlan, Guo Dean. Comparative study on Chromatographic Fingerprint of Salvia Miltiorrhiza in Xiangdan Injection produced by different manufacturers. Chinese Natural Medicine 2007...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com