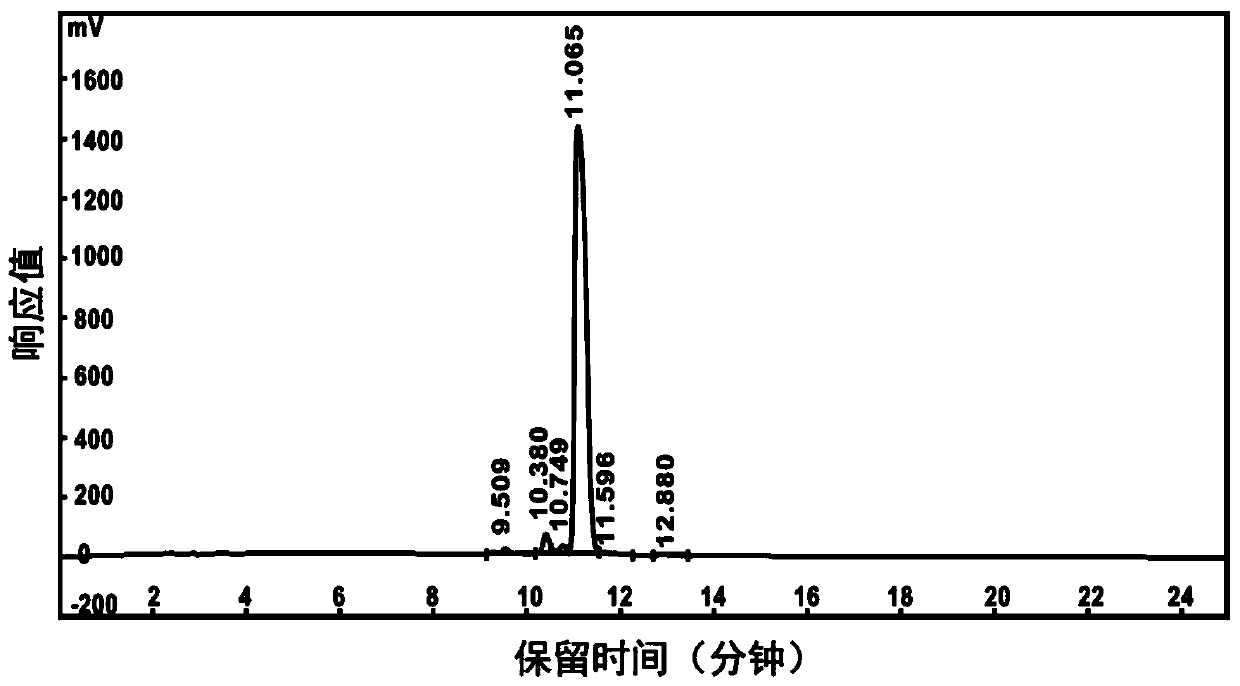
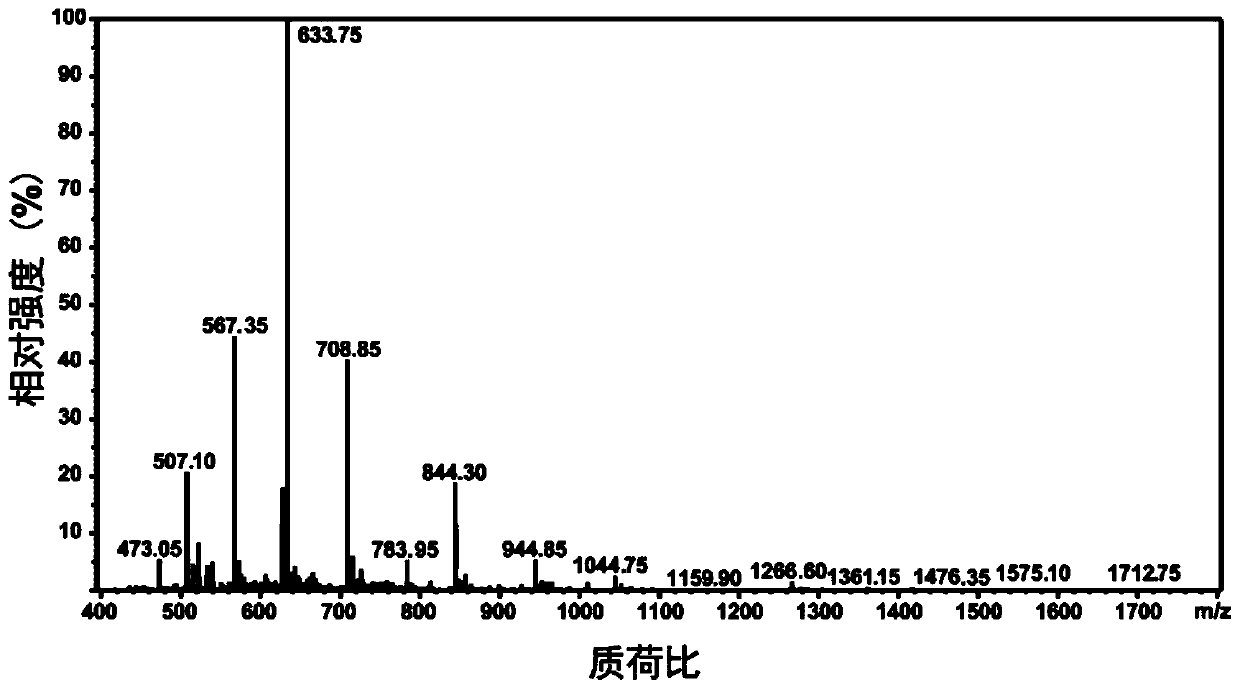
A kind of anti-tumor polypeptide nano drug and its preparation method and application
A nano-drug and anti-tumor technology, applied in the field of nano-materials, can solve the problems of molecular structure destruction and insufficient stability, and achieve the effect of uniform particle size, conducive to enrichment and improved targeting.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] In this example, the anti-tumor polypeptide nano drug was prepared by the following method:
[0080] The D-peptideantagonist ( D PPA-1) polypeptide (sequence from the amino terminal is: asparagine-tyrosine-serine-lysine-proline-threonine-aspartic acid-arginine-glutamine-tyrosine amino acid-histidine-phenylalanine) as a hydrophilic tumor immunotherapy polypeptide, according to the solid-phase synthesis method and the polypeptide purification method, the substrate for MMP-2 response, 2 glycines and 2 leucines are composed Hydrophobic peptides linked to tumor immunotherapy peptides D On PPA-1, a tripeptide formed by 3 lysines is connected to the amino terminal of the hydrophobic polypeptide, and then 4 3-(diethyl)s are connected through the amino groups on the lysines (including the terminal amino group and the side chain amino group). Amino)propylthioisocyanate functional molecule (DEAP) to obtain an amphiphilic tumor immunotherapy polypeptide coupled with an acid-respo...
Embodiment 2
[0092] The purpose of this example is to determine the morphology and particle size of self-assembled nanoparticles of anti-tumor polypeptide nano-drugs in a slightly acidic solution.
[0093] The pH value of the anti-tumor polypeptide nano drug system sample obtained in Example 1 was adjusted to 6.8, and after standing at room temperature for 2 hours, the morphology was observed with a transmission electron microscope and the particle size distribution was measured with a laser particle size analyzer. Such as Figure 4A and Figure 4B shown. Figure 4A It is a transmission electron microscope picture, in acidic solution, the polypeptide has a nano-spherical structure, Figure 4A The particle size distribution diagram can be drawn, the particle size distribution is 40-200nm, and the average particle size is 100nm.
[0094] In addition, when the pH value of the anti-tumor polypeptide nano-drug system sample was adjusted to 6.7, 7.0, 7.1 and 7.2, the results similar to that o...
Embodiment 3
[0097] The purpose of this example is to determine the responsiveness of anti-tumor polypeptide nano-medicines to the acidic environment of tumors in vivo.
[0098] Take 1 mg of the amphiphilic anti-tumor polypeptide coupled with DEAP (DEAP-amphipathic anti-tumor polypeptide) obtained in step (4) of Example 1, and 0.1 mg tetramethylrhodamine-5-isothiocyanate fluorescent The molecule and 0.1 mg of the quencher molecule were dissolved together in 10 μL of dimethyl sulfoxide, then added to 1 mL of phosphate buffer with a pH value of 7.4, and the mixture was sonicated for 2 min in an ultrasonic cleaner with a power of 100 W. After the sonication, the sample was left to stand at room temperature for 2 hours, then centrifuged at 10,000g for 5 minutes, and the supernatant was taken to obtain DEAP-anti-tumor polypeptide nano-drug self-assembled nanoparticles loaded with fluorescent molecules and quencher molecules at the same time. It is a relatively uniform and stable spherical struc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com