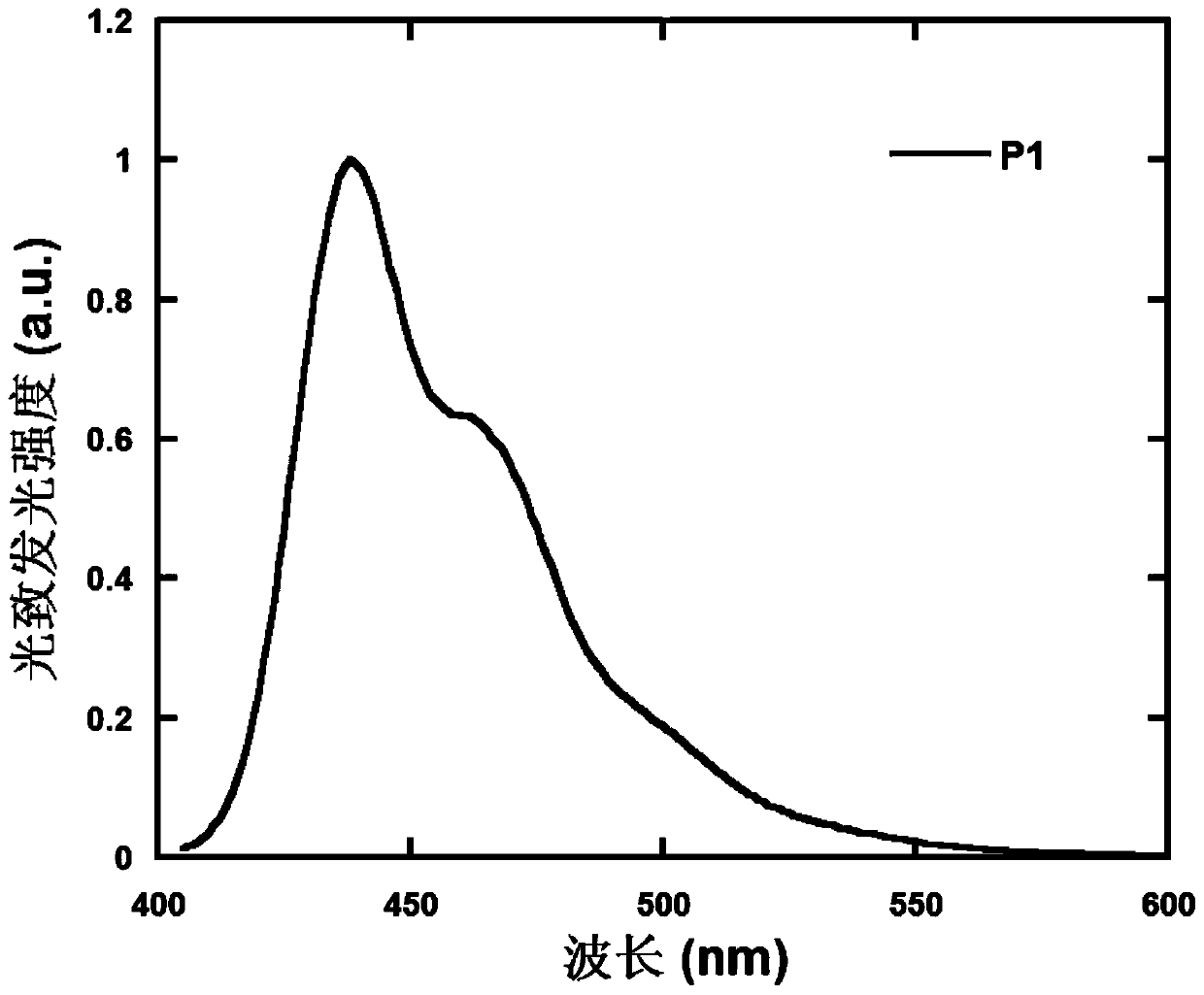
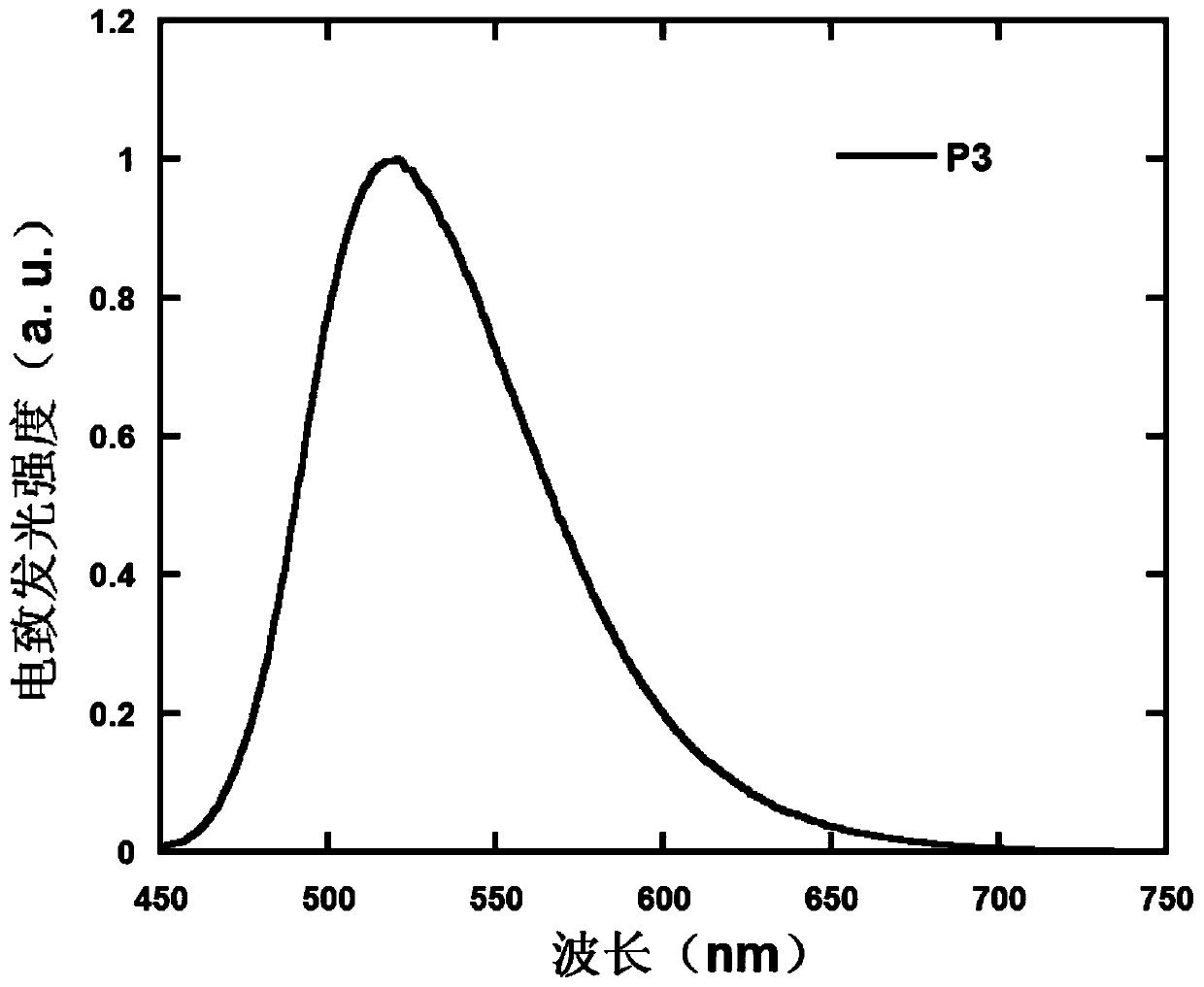
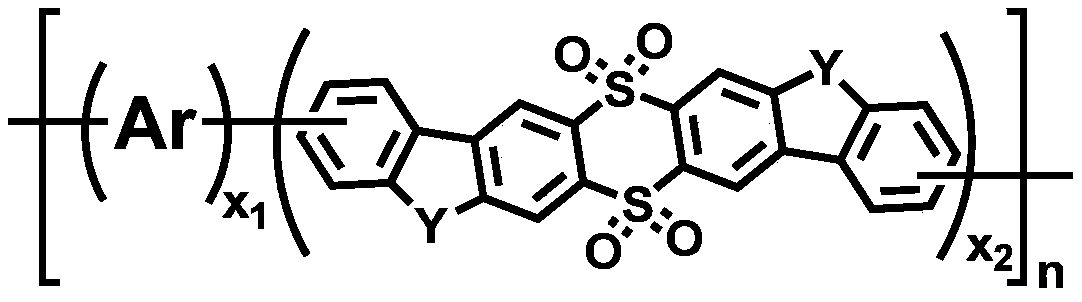
Polymer containing 9,9,10,10-tetraoxo-thianthracene seven-membered fused ring unit, preparation method and application thereof
A technology of polymer and thianthrene, which is applied in the fields of electrical components, semiconductor/solid-state device manufacturing, and electric solid-state devices, etc., can solve the problems of limiting the electroluminescence performance of polymer light-emitting materials, and achieve the improvement of electron transport performance and electron injection ability, the effect of improving carrier mobility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: the preparation of compound M1
[0042] (1) Preparation of compound 1
[0043] Under nitrogen protection, add 4-bromo-2 iodothiophenol (3.15g, 10mmol), palladium acetate (0.13mg, 0.5mmol), triphenylphosphine (0.26g, 1mmol) and tert-butyl Sodium alkoxide (3.85g, 40mmol) and 60mL of anhydrous toluene were heated to 80°C for 24 hours. After the reaction solution was cooled, the product was extracted with dichloromethane, and the organic phase was washed three times with saturated brine. The solvent was spin-dried, and the crude product was purified by column chromatography using petroleum ether as an eluent to obtain 2.69 g of a white solid with a yield of 72%. 1 HNMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0044] (2) Preparation of Compound 2
[0045] Under nitrogen protection, compound 1 (3.74g, 10mmol), pinacol diborate (6.35g, 25mmol), [1,1'-bis(diphenylphosphino)ferrocene ] Pall...
Embodiment 2
[0056] Embodiment 2: the preparation of compound M2
[0057] (1) Preparation of Compound 6
[0058] Under nitrogen atmosphere, compound 2 (4.68g, 10mmol), 5-bromo-2-iodonitrobenzene (9.84g, 30mmol), potassium carbonate (3.45g, 25mmol), tetrakis(triphenyl Phosphine)palladium (0.58g, 0.5mmol), 12mL of deionized water and 50mL of toluene were heated to 80°C for 12 hours. After the reaction was completed, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and the organic phase solvent was removed, and the crude product was purified by column chromatography using petroleum ether as eluent to obtain 4.07 g of a yellow solid with a yield of 66%. 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0059] (2) Preparation of Compound 7
[0060] Under nitrogen protection, compound 6 (6.16 g, 10 mmol) and 50 mL of triethyl phosphite were added into a 150 ...
Embodiment 3
[0067] Embodiment 3: the preparation of compound M3
[0068] (1) Preparation of Compound 9
[0069] Under a nitrogen atmosphere, compound 2 (4.68g, 10mmol), 2-iodonitrobenzene (7.47g, 30mmol), potassium carbonate (3.45g, 25mmol), tetrakis(triphenylphosphine) palladium were added to a 150mL two-necked flask (0.58g, 0.5mmol), 12mL deionized water and 50mL toluene, heated to 80°C for 12 hours. After the reaction was completed, the product was extracted with dichloromethane, washed three times with saturated aqueous sodium chloride solution, and the organic phase solvent was removed, and the crude product was purified by column chromatography using petroleum ether as eluent to obtain 3.44 g of a yellow solid with a yield of 75%. 1 H NMR, 13 The results of CNMR, MS and elemental analysis showed that the obtained compound was the target product.
[0070] (2) Preparation of Compound 10
[0071] Under nitrogen protection, compound 9 (4.59 g, 10 mmol) and 50 mL of triethyl phosphit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
| luminance | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com