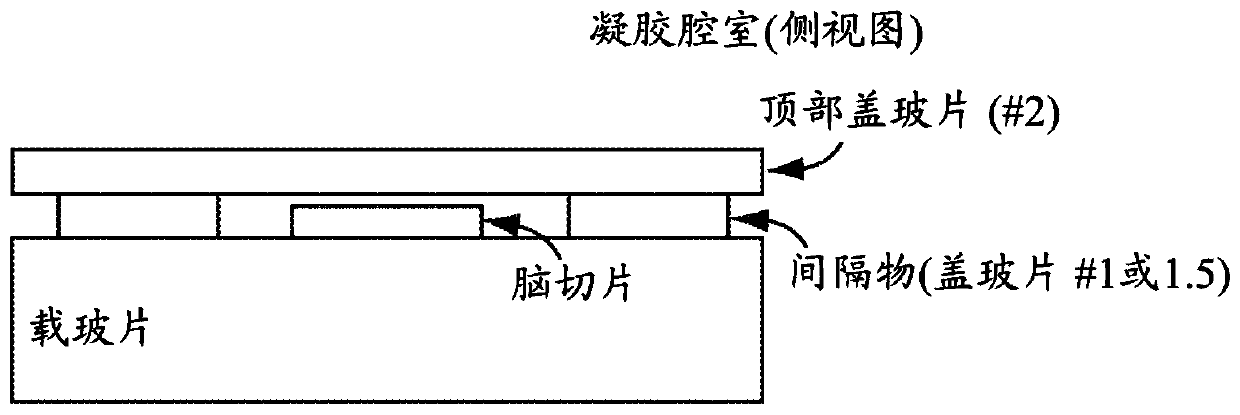
protein retention extension microscopy
A protein and fluorescent protein technology, applied in the field of protein retention extended microscopy, can solve the problems of customized synthesis obstacles, inability to customize reagents, and inability to image fluorophores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0096] raw material solution
[0097] 4% paraformaldehyde
[0098] 4% Paraformaldehyde (from Electron Microscopy Science 16% stock)
[0099] 1x PBS
[0100] Quench solution (store at 4C for use over extended periods of time)
[0101] 1x PBS
[0102] 100mM Glycine
[0103] protein anchor solution
[0104] 1x PBS
[0105] 0.1 mg / mL 6-((acryloyl)amino)caproic acid, succinimidyl ester (acryloyl-X, SE)
[0106] Tissue Disruption Solution (Autoclaved Version)
[0107] 100mM Tris base
[0108] 1% sodium lauryl sulfate
[0109] 5% Triton X-100
[0110] Tissue Disruption Solution (Phospholipase Version)
[0111] 0.5x PBS
[0112] 0.1% Triton X-100
[0113] Phospholipase A1 (Sigma, L3295) 100U / mL
[0114] Phospholipase D (Enzo, BML-SE301-0025) 500U / mL
[0115] Antibody staining solution (stored at 4C, can be used for at least 1 month)
[0116] 1x PBS
[0117] 0.1% Triton X-100
[0118] 2% normal donkey serum
[0119] Monomer solution:
[0120]
[0121]
[012...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com