Synthetic method of kelp paper a
A technology of paper millet and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of time-consuming, harsh reaction conditions, cumbersome steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
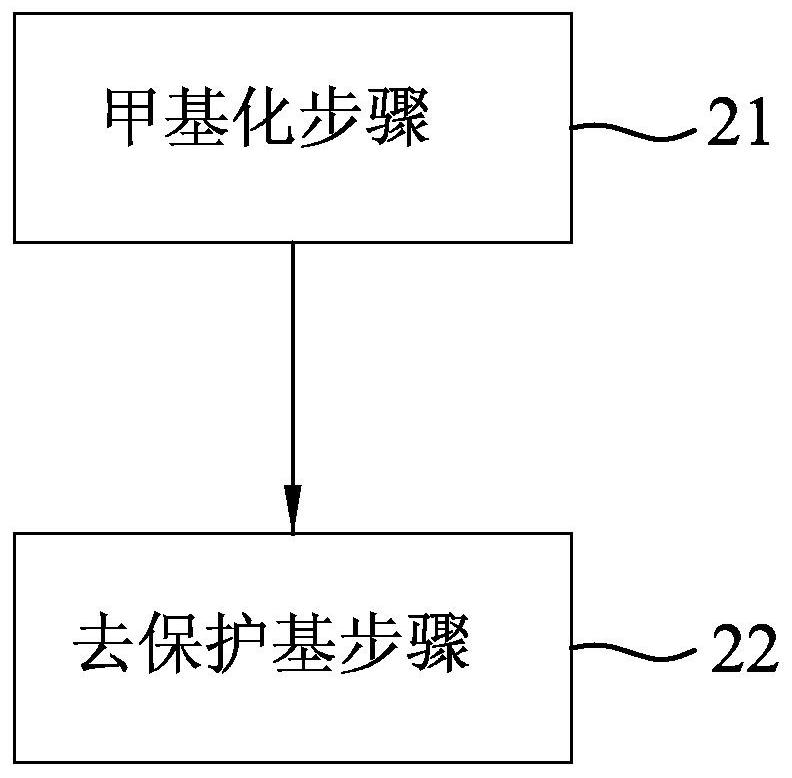
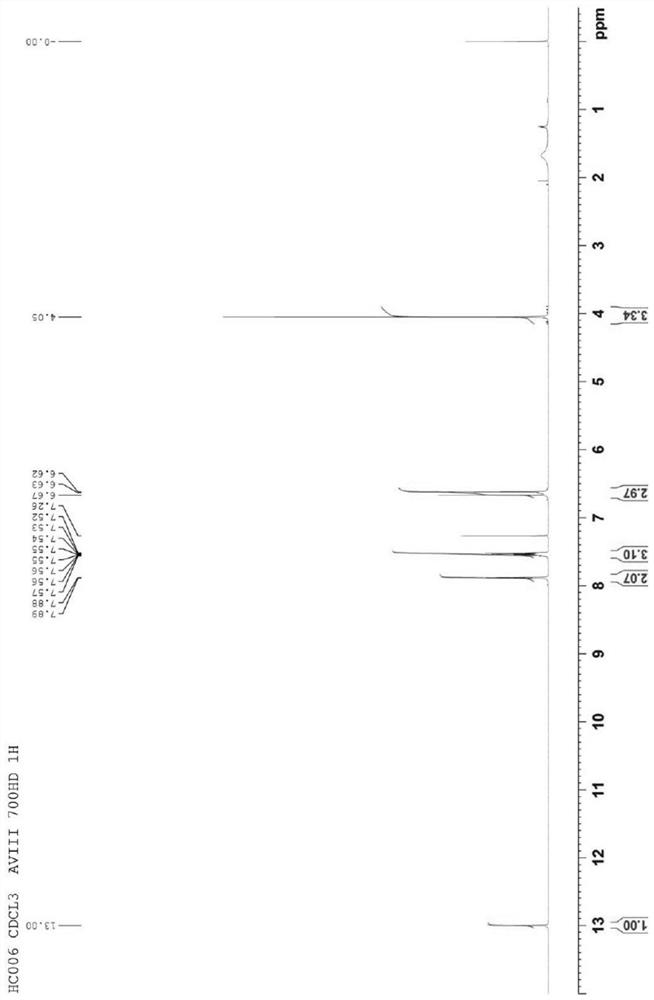
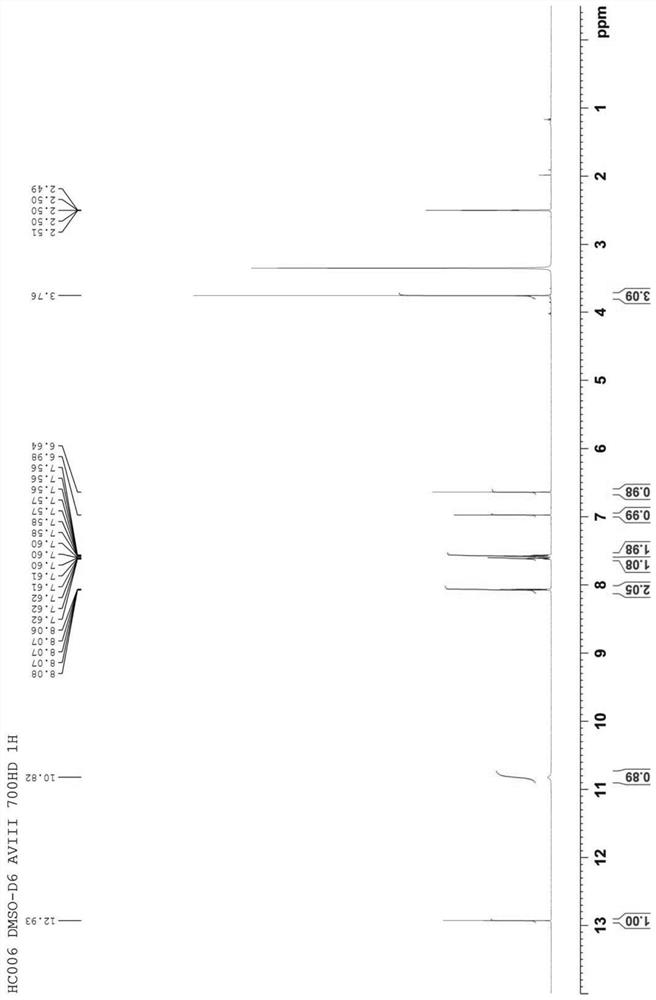
[0024] refer to figure 1 , an embodiment of the synthesis method of paperlanin A of the present invention, comprising a methylation step 21 and a protecting group removal step 22 .
[0025] In the methylation step 21, baicalin is used as a starting material, and the starting material is subjected to a methylation reaction in a first reaction solution composed of an inorganic base and a methylating reagent, so that 6 of the starting material is -Hydroxyl is methylated to give an intermediate.
[0026] It is well known to those skilled in the art that flavonoids (such as the structure of melaleucain A in this case) have a γ-pyrone ring that is easily cleaved under alkaline conditions. Therefore, generally in the process of synthesizing flavonoids or When utilizing flavonoids to react, all need to be under relatively severe conditions (for example, the reagents used need to remove water, and the reaction should be carried out under inert gas), so as to avoid the γ-pyrone ring in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com