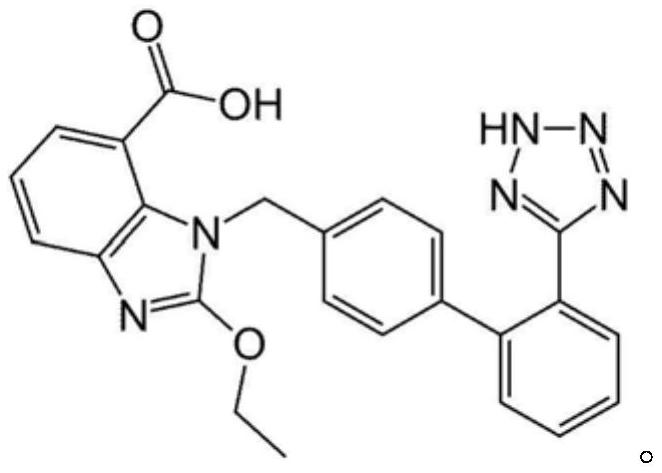
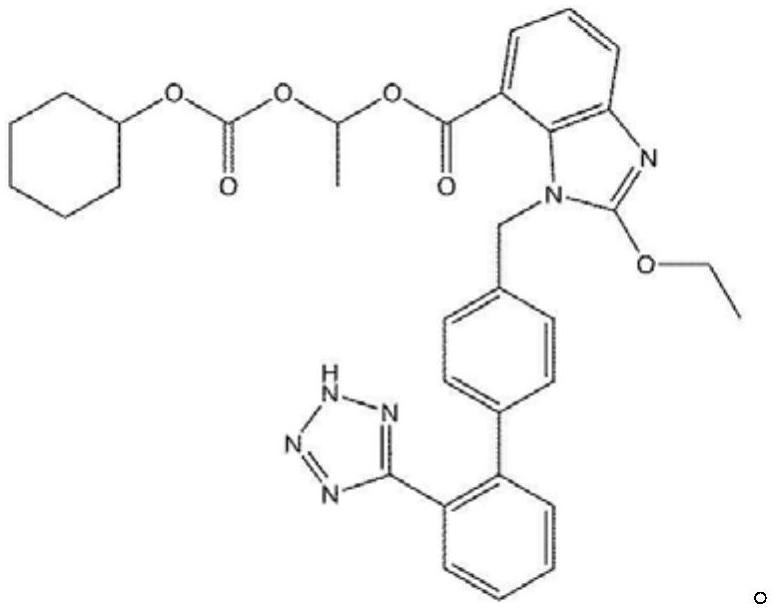
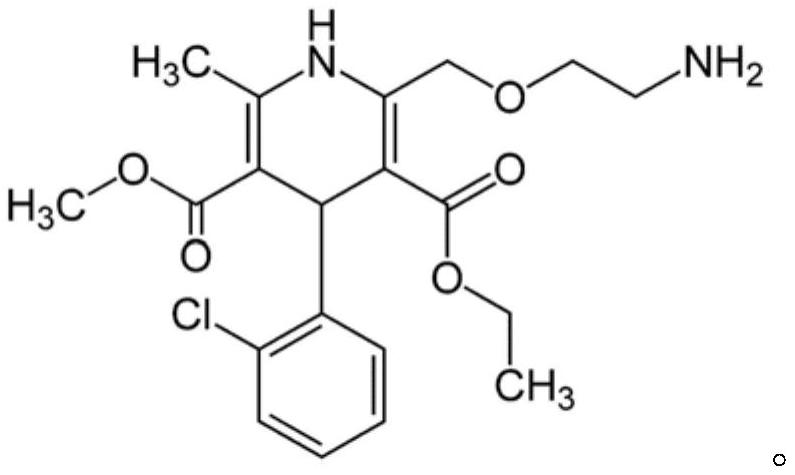
drug combination preparation
一种片剂、药学的技术,应用在制备该片剂领域,能够解决固有溶解度差、降低药物功效、低水溶性等问题,达到良好稳定性和溶解性、治疗或预防高血压、良好成片性和生产效率的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: the preparation of bilayer tablet
[0062] 6 g of hydroxypropylcellulose and 3 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 16 g of candesartan cilexetil, 65 g of microcrystalline cellulose, and 10 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare a mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0063] 79 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 1 g of magnesium stearate was added to obtain a lubricated mixture (1).
[0064] 5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 13.87 g of amlodipine besylate, 71.13 g of microcrystalline c...
Embodiment 2
[0068] Embodiment 2: the preparation of bilayer tablet
[0069] 6 g of hydroxypropylcellulose and 3 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 16 g of candesartan cilexetil, 65 g of microcrystalline cellulose, and 10 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare a mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0070] 79 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 1 g of magnesium stearate was added to obtain a lubricated mixture (1).
[0071] 2.5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 6.935 g of amlodipine besylate, 35.565 g of microcrystallin...
Embodiment 3
[0075] Embodiment 3: the preparation of bilayer tablet
[0076] 3 g of hydroxypropylcellulose and 1.5 g of polyethylene glycol 15-hydroxystearate were dissolved in purified water and ethanol to prepare a binding solution (1). 8 g of candesartan cilexetil, 32.5 g of microcrystalline cellulose, and 5 g of pregelatinized starch were uniformly mixed in a high-speed mixer to prepare mixture (1). The binding solution (1) is added to the mixture (1), granulated and then dried. Sieved granules (1) were obtained by sieving with a 20-mesh sieve in a semi-dry state and with a 30-mesh sieve in a dry state.
[0077] 39.5 g of silicified microcrystalline cellulose were added to the sieved granules (1 ) and mixed in a double cone mixer. Then, 0.5 g of magnesium stearate was added to obtain a lubricated mixture (1).
[0078] 2.5 g of hydroxypropylcellulose was dissolved in purified water and ethanol to prepare a binding solution (2). 6.935 g of amlodipine besylate, 35.565 g of microcrysta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com