A method of producing chimeric antigen receptor modified gamma delta t cells
A chimeric antigen receptor, cell technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1. A method for expanding γδ T cells
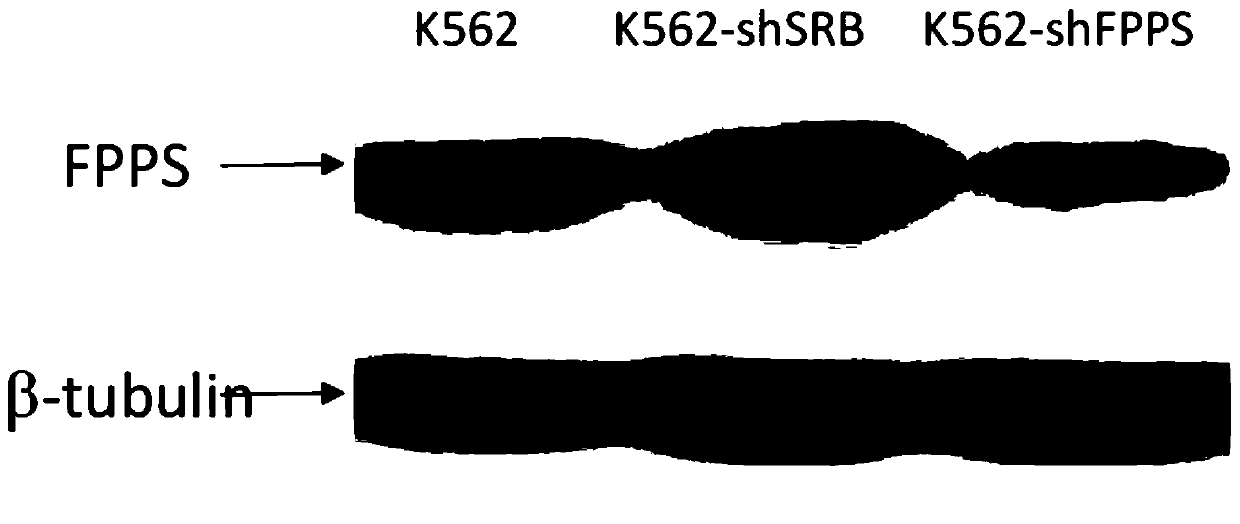
[0071] 1. Construction of K562-shFPPS tumor cell line with reduced expression of FPPS
[0072] 1. Construction of lentiviral recombinant plasmid
[0073] (1) Preparation of recombinant plasmid
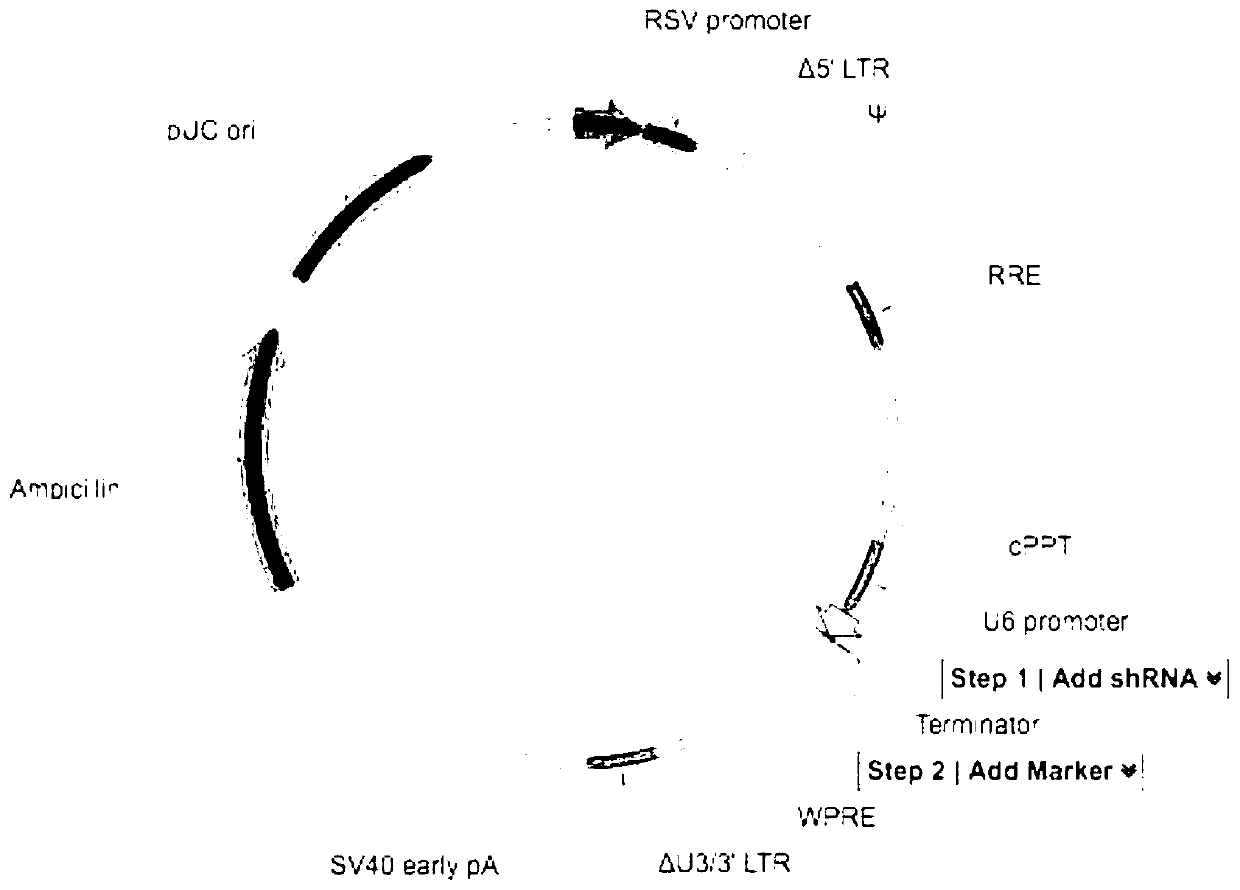
[0074] The U6-based shRNA construction system of the Vectorbuilder system of Saiye Biotechnology Co., Ltd. was used to construct the lentiviral recombinant plasmid. Specific steps are as follows:
[0075] Design and synthesize a specific shRNA coding sequence targeting FPPS (shFPPS) as the experimental group: CCAGCAGTGTTCTTGCAATATCTCGAGATATTGCAAGAACACTGCTGG (sequence 1); wherein, the bold 6bp sequence in the middle is the stem-loop sequence, its left 21bp is the sense sequence, and the right The 21bp sequence is an antisense sequence; at the same time, the following Scramble-shRNA coding sequence (shSRB) was synthesized as a negative control group: CCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG; among them, the 6bp sequence in ...
Embodiment 2
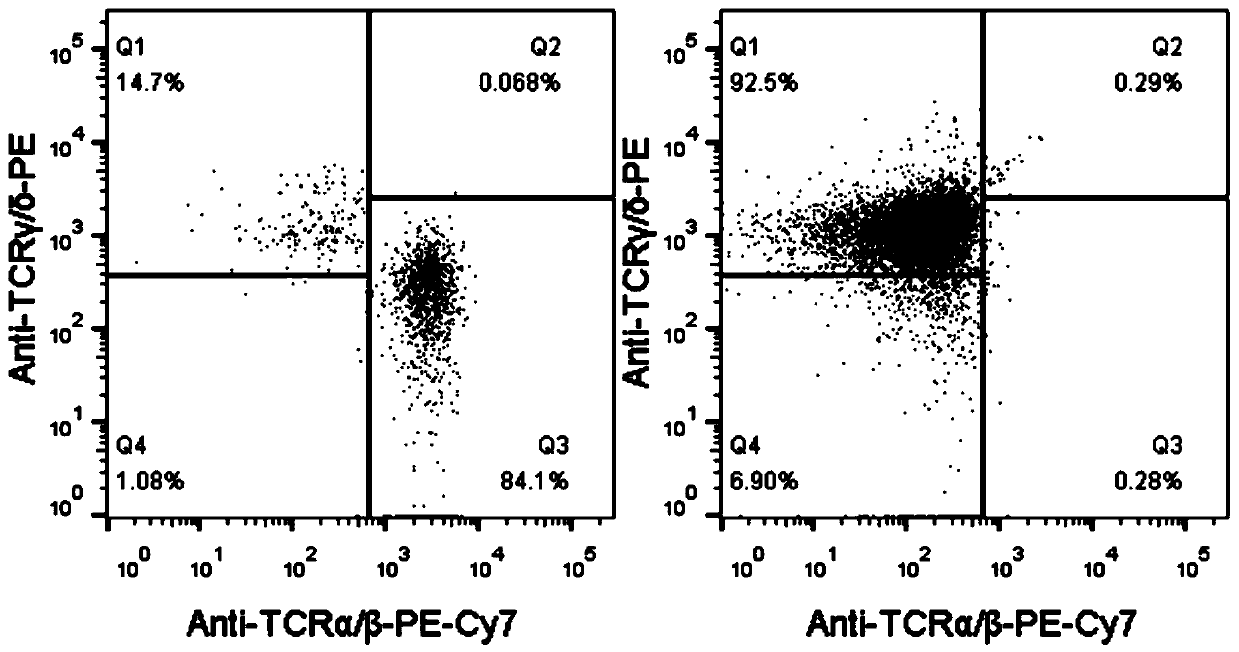
[0115] Example 2, Production method of CAR-γδ T cells
[0116] 1. Preparation of CAR22 lentiviral particles
[0117] The present invention uses the CAR structure to genetically modify γδ T cells, and the CAR structure from the amino terminal to the carboxyl terminal is as follows: ScFv(CD22)-Hinge(CD8)-TM(CD8)-CD137-CD3ζ, that is, from the amino terminal to the carboxyl terminal The sequence is: single-chain variable region derived from CD22 monoclonal antibody (clone number: M971), CD8a hinge region and transmembrane region, CD137 signal domain and CD3ζ chain intracellular region. Its amino acid sequence is shown in sequence 3 of the sequence listing, and its nucleotide sequence is shown in sequence 4 of the sequence listing. Insert the DNA molecule shown in Sequence 4 into the Senl_pLenti-EF1 vector (the Senl_pLenti-EF1 vector is a vector obtained by adding restriction sites PacI and SpeI on both sides of the original plasmid cloning site, and the original plasmid name is L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com