Applications of phosphodiesterase type 4 inhibitors in preparation of novel anti-inflammatory drugs
A technology of phosphodiesterase and anti-inflammatory drugs, applied in the field of medicine, can solve problems such as vermilion dilactone that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] The following describes the substantive content of the present invention in detail in conjunction with the drawings and embodiments, but does not limit the protection scope of the present invention.
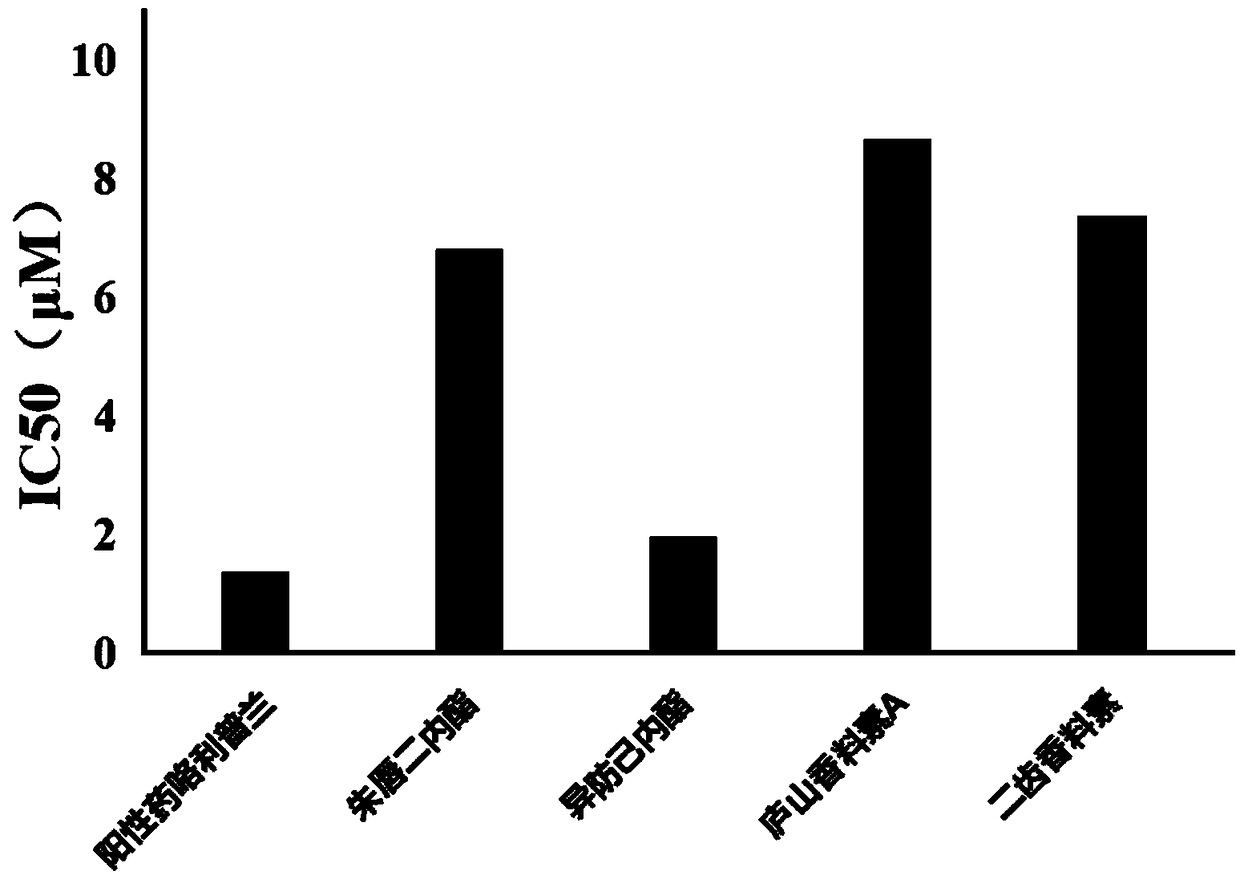
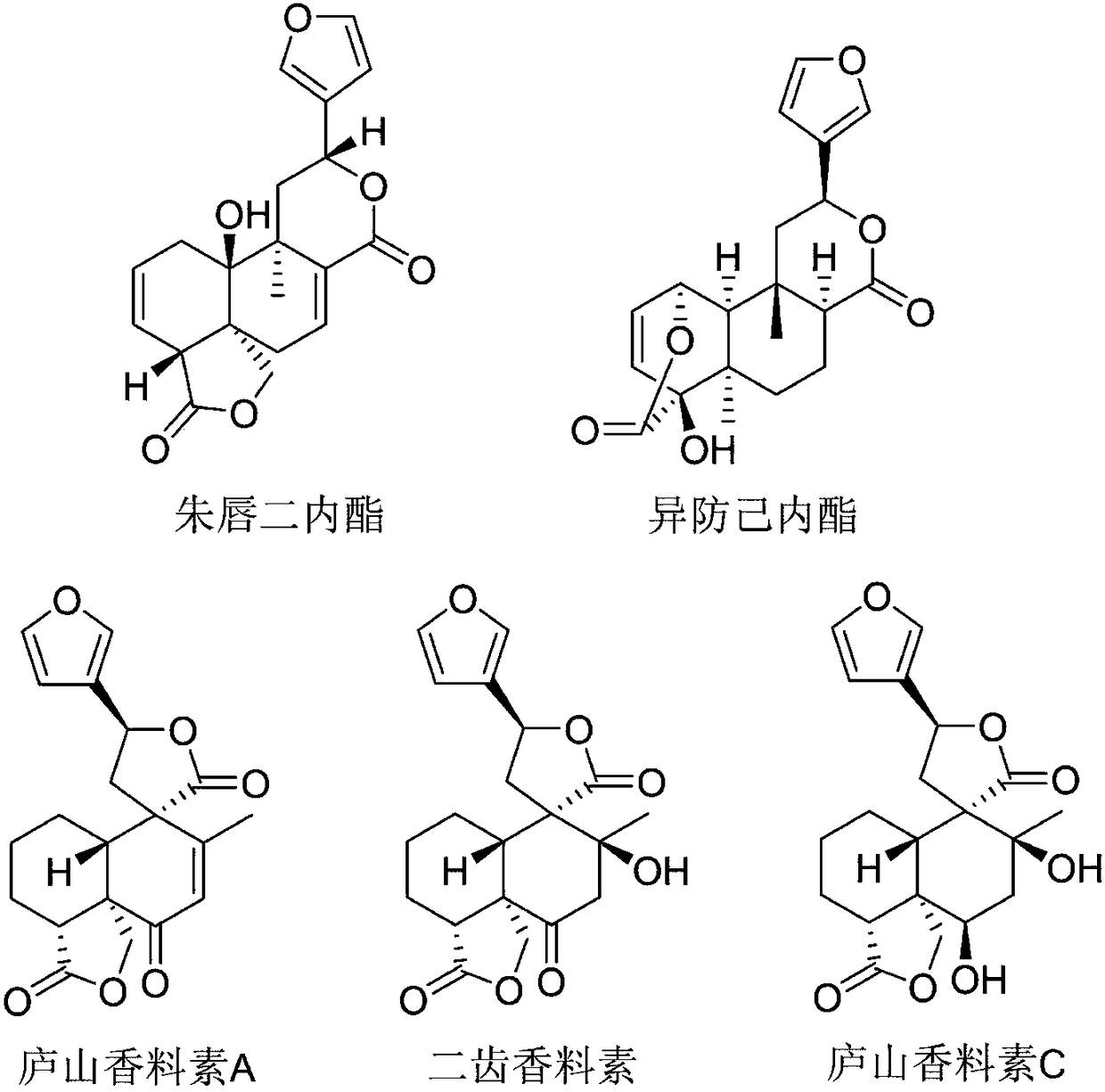
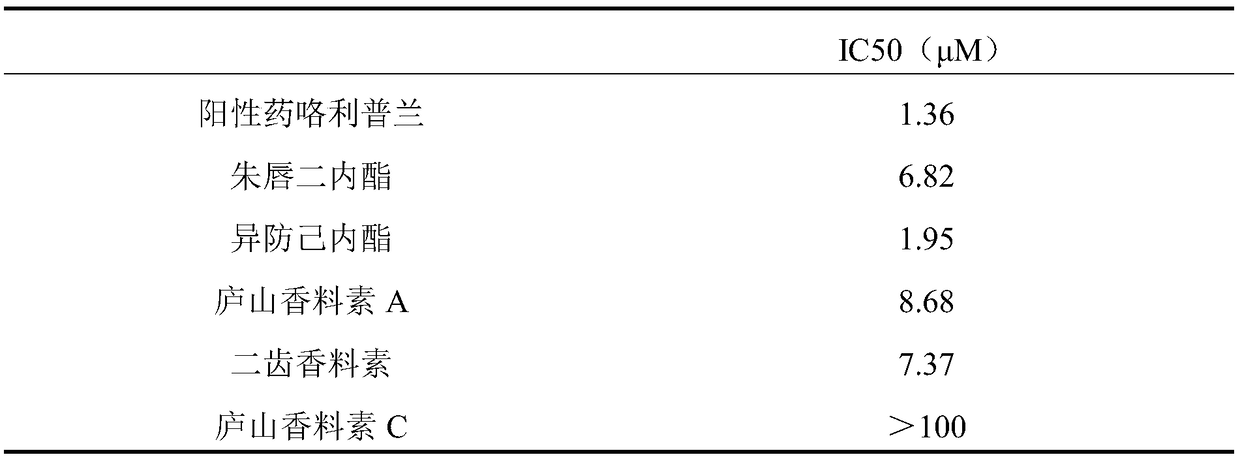
[0018] According to the literature method (Liu Junfeng et al., RP-HPLC determination of phosphodiesterase 4 inhibitory activity of bitter wood alkaloids in vitro, Chinese modern traditional Chinese medicine, 2009 03 issue) determination of vermilion dilactone, isofantrolactone, and Lushan spicein A Inhibition of phosphodiesterase 4 by bidentate spices and Lushan spices C.
[0019] 1. Experimental materials and instruments
[0020] Vermilion Dilactone, Isopredylactone, Lushan Perfume A, Bidentate Perfume and Lushan Perfume C are self-made or purchased, and the purity is not less than 98%. cAMP and the positive drug Rolipram were purchased from Sigma. Lymphocyte separation medium was purchased from Nanjing Jiancheng Biotech. Blood samples were collected from healthy comme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com