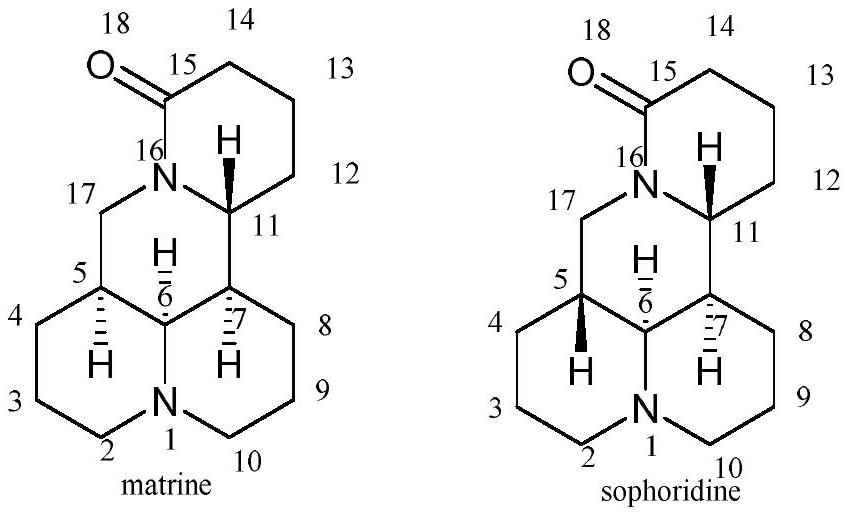
Matrine derivatives and sophoridine derivatives containing aryl amidine or aminoquinoline structure and preparation method thereof
A technology for matrine and sophoridine, which is applied to matrine derivatives and sophoridine derivatives containing aryl amidine or aminoquinoline structures and their preparation fields, can solve the problems of not having too much activity and the like, and achieves the Strong inhibitory effect, high antitumor activity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
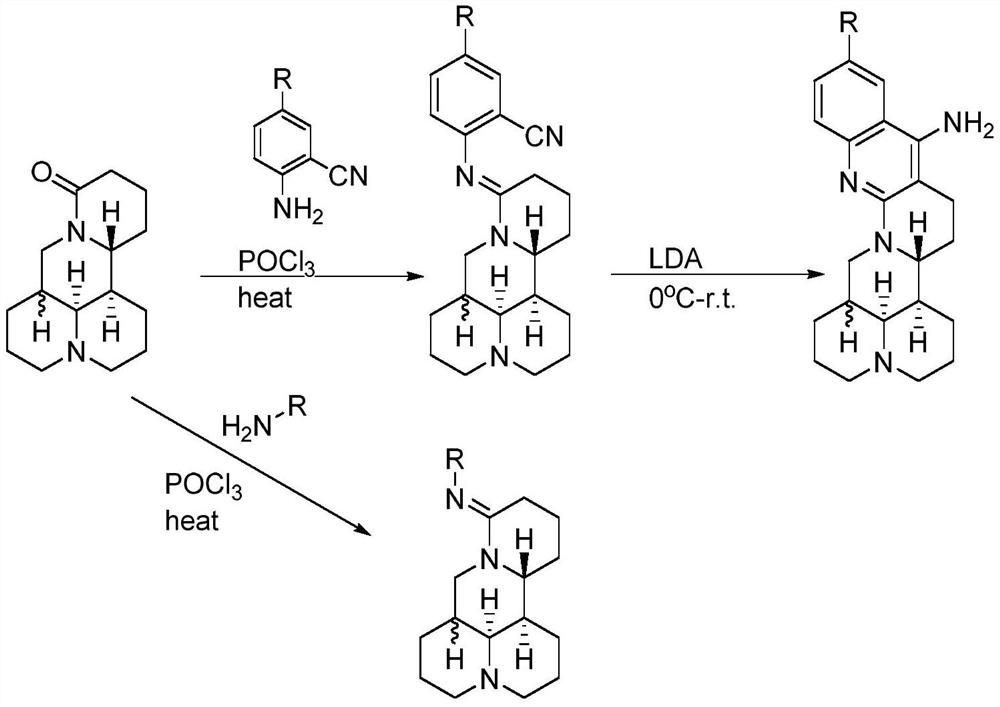
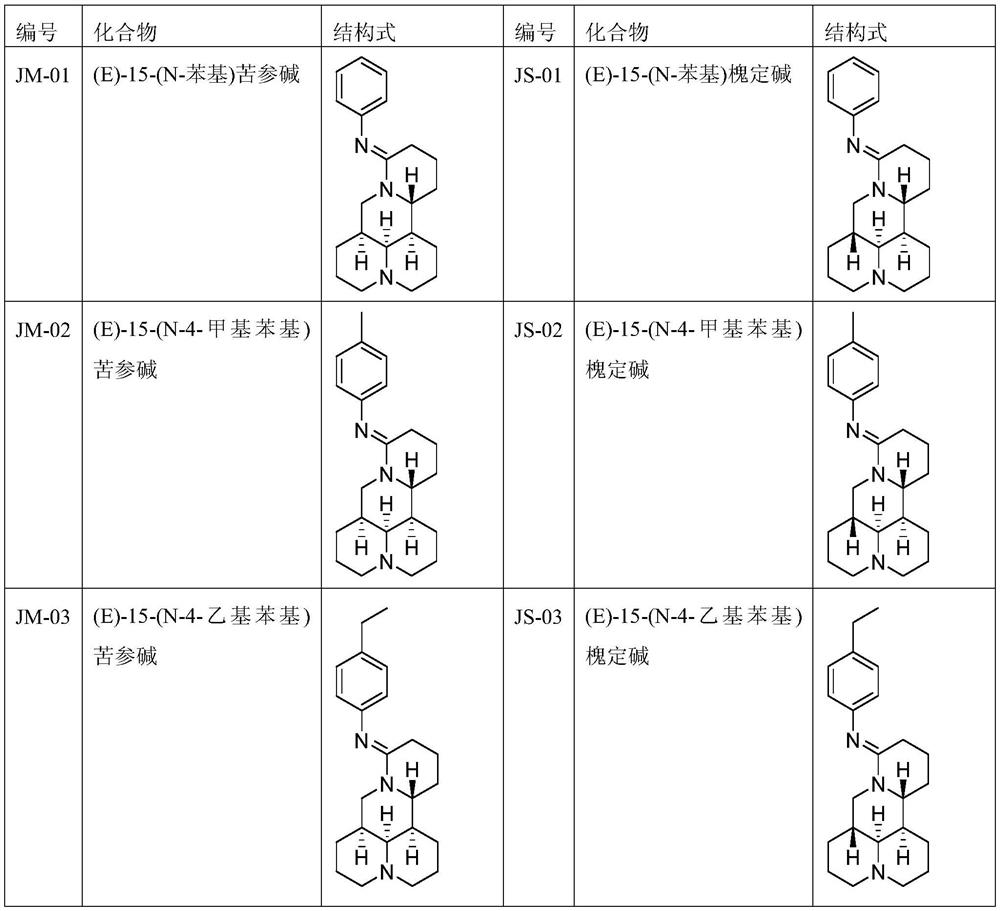
[0014] The preparation method of compound JM-01, the steps are as follows:
[0015] (1) Dissolve 5 mmol (1.24 g) of matrine in 30 ml of dichloromethane (DCM), and add 2 eq (10 mmol) of phosphorus oxychloride (POCl) dropwise under stirring at room temperature 3 ), after the solution changed from colorless to light yellow, put it in an oil bath at 60°C and stir and reflux for 1-3 hours until the solution turned brownish red.
[0016] (2) Return the solution to room temperature, and add 1 to 1.5 eq (5 to 7.5 mmol) of aniline in DCM dropwise while stirring, and place the solution in an oil bath at 60° C. to stir and reflux for 12 hours after the dropping.
[0017] (3) After the reaction is completed, return the solution to room temperature, slowly add cold NaOH solution or NaHCO dropwise under stirring 3 Saturated solution, neutralized the pH of the reaction solution to 8-9, stirred for 10 min, extracted three times with water and DCM, and combined the organic phases.
[0018] (...
Embodiment 2
[0149] The preparation method of compound JM-30, the steps are as follows:
[0150] (1) Dissolve 5 mmol (1.24 g) of matrine in 30 ml of dichloromethane (DCM), and add 2 eq (10 mmol) of phosphorus oxychloride (POCl) dropwise under stirring at room temperature 3 ), after the solution changed from colorless to light yellow, put it in an oil bath at 60°C and stir and reflux for 1-3 hours until the solution turned brownish red.
[0151] (2) Return the solution to room temperature, and add 1 to 1.5 eq (5 to 7.5 mmol) of a DCM solution of 2-cyanoaniline dropwise while stirring, and continue to place the solution in a 60°C oil bath for stirring Reflux for 12h.
[0152] (3) After the reaction is completed, return the solution to room temperature, slowly add cold NaOH solution or NaHCO dropwise under stirring 3 Saturated solution, neutralize the pH of the reaction solution to 8-9, stir for 10 min, extract with water and DCM three times, and combine the organic phases.
[0153] (4) Co...
Embodiment 3
[0185] The present invention also includes the application of the imine and aminoquinoline derivatives in the preparation of antitumor drugs. Preferably, the tumors are liver cancer (HepG2) and cervical cancer (HeLa).
[0186] Experimental method: the above-mentioned imine and aminoquinoline derivatives were dissolved and diluted with dimethyl sulfoxide (DMSO) to form a 100um mother solution. During the experiment, the mother solution was diluted with the culture medium to the concentration required for the test. Take a bottle of logarithmic growth cells, digest with trypsin, centrifuge, resuspend, and count under a microscope. According to the counting results, the cells were seeded in a 96-well plate at a density of 5000 cells per well (100 ul medium), and placed in an incubator for 24 hours. After the cells adhered to the wall, the medium was carefully sucked away, and the medium containing different concentrations of the drug to be tested was added to culture for 44 hour...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com