A pharmaceutical combination preparation of recombinant human vascular endothelial growth factor receptor-antibody fusion protein
A growth factor receptor and fusion protein technology, which is applied in the field of recombinant human vascular endothelial factor receptor-antibody fusion protein drug combination preparation and its preparation, can solve problems such as complex structure, and achieve the effect of inhibiting aggregation and precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
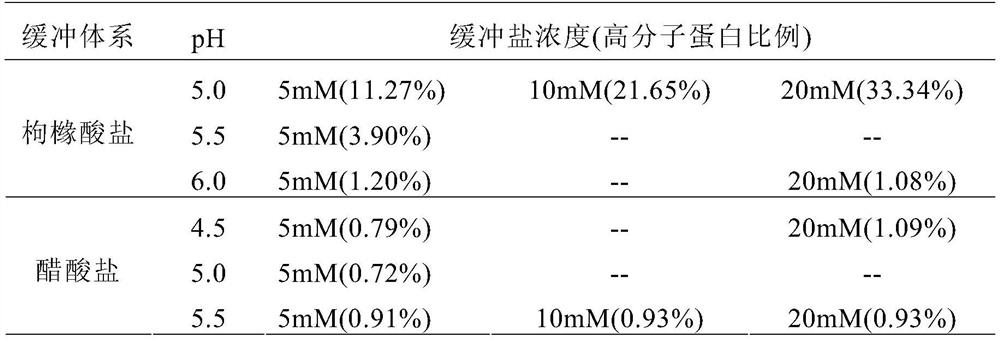
[0076] Example 1 Stability comparison of different buffer systems
[0077] The high-concentration human recombinant vascular endothelial growth factor receptor-antibody fusion protein was replaced by G-25 desalting to the buffer salt system to be screened (as shown in Table 1), and the protein concentration was adjusted to about 10mg / mL, sterilized and filtered for later use.
[0078] The prepared samples to be studied were stored under high temperature and pressure (40°C ± 2°C), and 2 weeks later, samples were taken and sent to the SEC-HPLC test items. The test results are shown in Table 1.
[0079] Table 1 Stability comparison of different buffer systems (SEC-HPLC polymer impurity content)
[0080]
[0081] The results showed that in the citrate buffer system (pH 5.0-6.0), the higher the pH value, the more stable it was; in the buffer system concentration range of 5-20 mM, the stability of the recombinant fusion protein decreased with the increase of the buffer concentr...
Embodiment 2
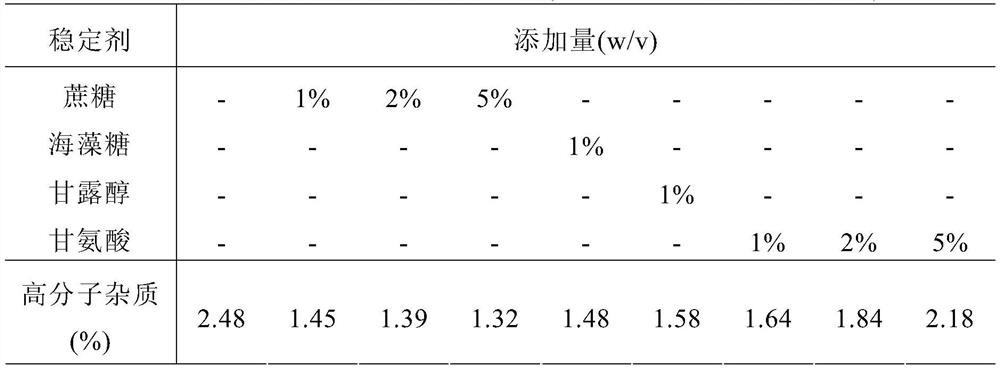
[0082] Example 2 Comparison of protective effects of different stabilizers
[0083] In the buffer system of 5 mM acetate (pH 5.7), the protective effect of each stabilizer was compared, and the screened stabilizers included sugar alcohol, sodium chloride and amino acid.
[0084] High-concentration human recombinant vascular endothelial growth factor receptor-antibody fusion protein was pre-exchanged to a 5 mM acetate buffer system, each formulation was prepared by adding high-concentration stock solutions of each stabilizer, and the protein concentration was adjusted to about 10 mg / mL.
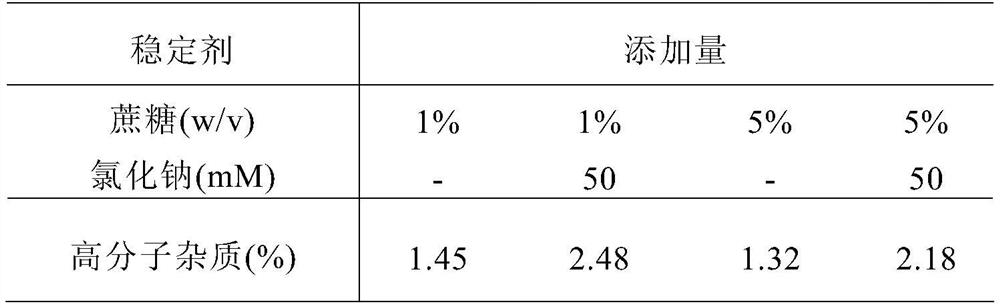
[0085] The prepared samples to be studied were stored under high temperature and pressure (40°C ± 2°C), and 4 weeks later, samples were taken and sent to the SEC-HPLC test items. The test results are shown in Table 2 and Table 3.
[0086] Table 2 Comparison of protection effects of different stabilizers (SEC-HPLC polymer impurities content)
[0087]
[0088] Table 3 Comparison of protectio...
Embodiment 3
[0092] Example 3 Recombinant Human Vascular Endothelial Growth Factor Receptor-Antibody Fusion Protein Drug Combination Preparation
[0093] The preparation is prepared according to the following formula, and the amino acid sequence of the recombinant fusion protein is shown in SEQ ID NO.: 1.
[0094] prescription
[0095] Recombinant human vascular endothelial growth factor receptor-antibody fusion protein 10mg / mL Acetate buffer system (glacial acetic acid + sodium acetate) 5mM sucrose 85g / L pH 5.7
[0096] The solvent is sterile water for injection.
[0097] The semi-finished products were aseptically distributed into vials (0.2 mL / bottle), and covered with bromobutyl rubber stoppers and aluminum caps to obtain finished products.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com