Dysprosium complexes mixed with 8-hydroxyquinoline derivatives and 1,10-phenanthroline and their preparation methods and applications
A technology of hydroxyquinoline and o-phenanthroline, applied in the field of magnetic materials, achieves the effects of low cost, good repeatability, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: the preparation of complex 1
[0035]
[0036] The specific synthesis method is: 0.1mmol of 2-methyl-5,7-dibromo-8-hydroxyquinoline (0.0317g) and 0.1mmol of Dy(NO 3 ) 3 ·6H 2 O (0.0460g), added to a Pyrex tube with one end closed and about 18cm long, then added 0.1mmol of 1,10-phenanthroline (0.0182g), and then added 0.4mL of 1,4-dioxane and 1.1mL of water (the volume ratio of 1,4-dioxane and water is 1:2.75), and then with Et 3 N (about 10 μL) to adjust the pH of the system to 5.5, vacuumize the Pyrex tube, and seal the other end; put the sealed Pyrex tube in an oven at 90°C for 72 hours, take it out, and slowly cool it to room temperature to observe At the bottom of the Pyrex tube, yellow blocky crystals precipitated. Yield 32.60% (based on Dy).
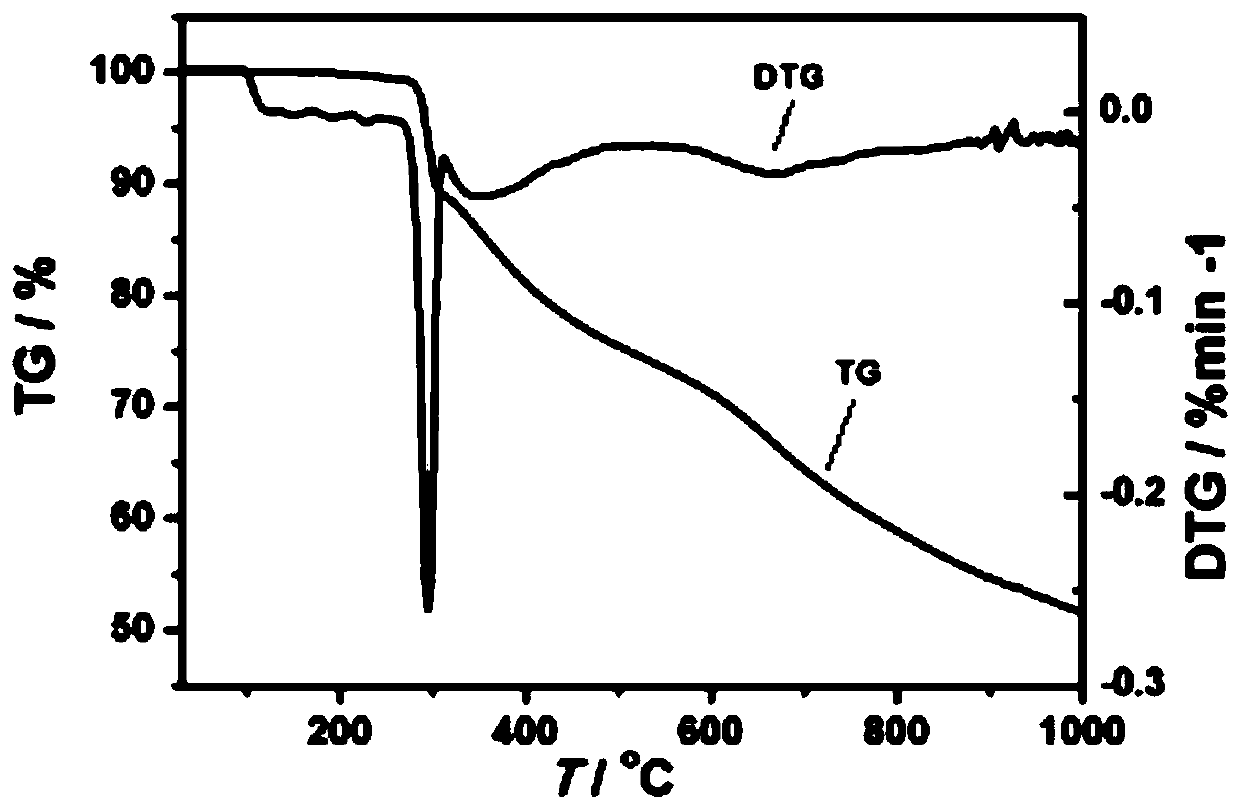
[0037] The product obtained in this embodiment is characterized:
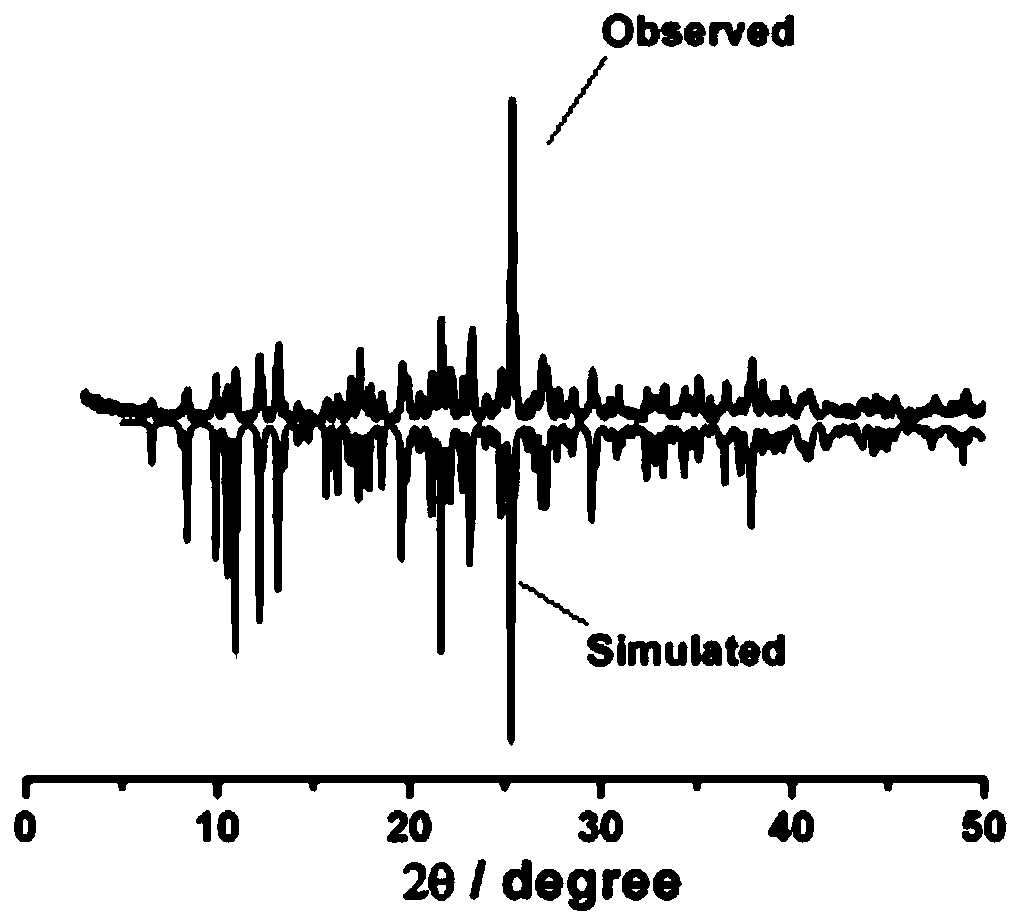
[0038] 1) Single crystal diffraction and structure analysis:
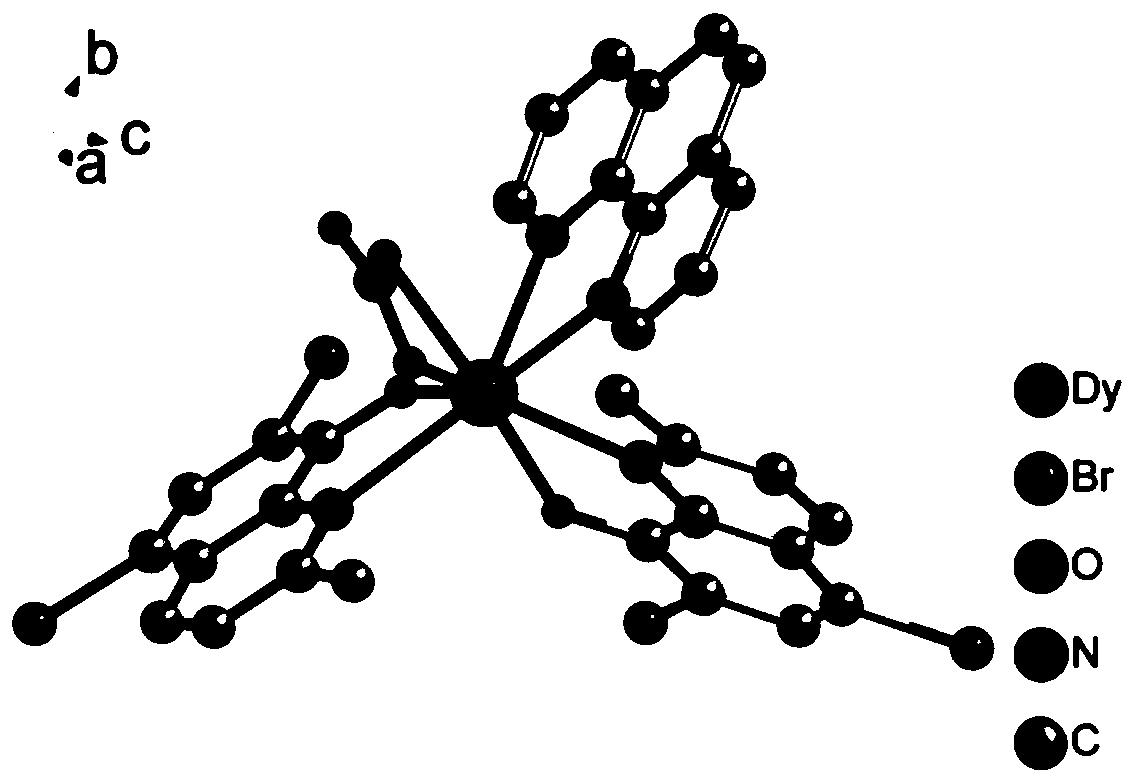
[0039] Select yellow blocky crystals of moderate size and place them o...
Embodiment 2
[0056] Embodiment 2: the preparation of complex 1
[0057] Repeat Example 1, the difference is:
[0058] 1) In the composition of the mixed solvent, the volume ratio of 1,4-dioxane and water is 1:4;
[0059] 2) adjusting the pH of the resulting solution to 5.8 with triethylamine;
[0060] 3) The reaction is carried out at 60°C.
[0061] After the reaction was completed, it was slowly cooled to room temperature, and yellow blocky crystals were precipitated at the bottom of the Pyrex tube. Yield 32.60% (based on Dy).
[0062] The product obtained in this example was analyzed by single crystal diffraction, and it was determined that the obtained yellow blocky crystal was the complex 1 described in the present invention, that is, Dy Ⅲ (L1) 2 (L2)(NO 3 ), where L1 is 2-methyl-5,7-dibromo-8-hydroxyquinoline with a hydroxyl hydrogen atom removed, with a unit negative charge; L2 is 1,10-phenanthroline.
Embodiment 3
[0063] Embodiment 3: the preparation of complex 1
[0064] Repeat Example 1, the difference is:
[0065] 1) In the composition of the mixed solvent, the volume ratio of 1,4-dioxane and water is 1:2;
[0066] 2) adjusting the pH of the resulting solution to 6.0 with triethylamine;
[0067] 3) The reaction is carried out at 90°C.
[0068] After the reaction was completed, it was slowly cooled to room temperature, and yellow blocky crystals were precipitated at the bottom of the Pyrex tube. Yield 32.85% (based on Dy).
[0069] The product obtained in this example was analyzed by single crystal diffraction, and it was determined that the obtained yellow blocky crystal was the complex 1 described in the present invention, that is, Dy Ⅲ (L1) 2 (L2)(NO 3 ), where L1 is 2-methyl-5,7-dibromo-8-hydroxyquinoline with a hydroxyl hydrogen atom removed, with a unit negative charge; L2 is 1,10-phenanthroline.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap