Fully-humanized monoclonal antibody and preparation method and application thereof
A monoclonal antibody, fully humanized technology, applied in the fields of biomedicine and genetic engineering, which can solve the problems of low specificity, limitation, pathogenic pollution, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
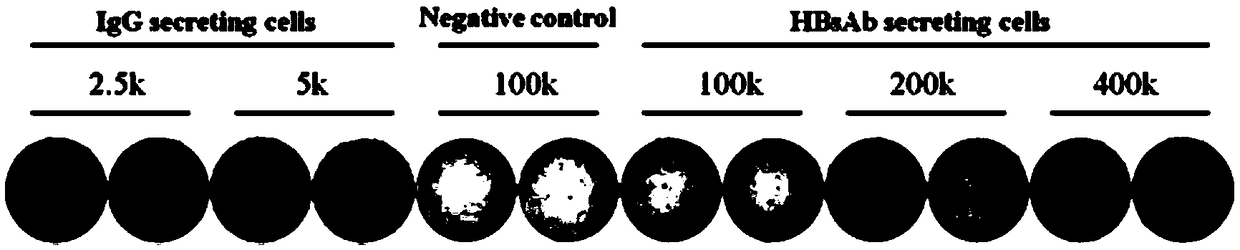
[0036] Example 1: Preparation of Hepatitis B virus surface antigen-specific monoclonal antibody
[0037] 1) Take 20 mL of peripheral blood from healthy individuals who have been vaccinated against hepatitis B vaccine within the recent six months to obtain B lymphocytes that secrete surface antibodies.
[0038] Take 20mL of fresh anticoagulated blood and separate the mononuclear cells in the peripheral blood. Using Pharmingen IMag TM (BD) Negative selection of B lymphocytes, the specific operation is as follows: Use 1x BD IMag TM Resuspend mononuclear cells in buffer; add biotinylated antibody Hunan B Lymphocyte Enrichment Cocktail, incubate at room temperature for 15 minutes; use 10 times the volume of 1xBDIMag TM Wash cells with buffer, centrifuge at 300×g for 7 minutes, aspirate all supernatant; vortex magnetic beads BDIMag TM Streptavidin Particles Plus-DM, add magnetic beads to cell suspension; mix thoroughly, incubate at room temperature for 30 minutes; add 1XBD IMag ...
Embodiment 2
[0047] Example 2: Variable region sequencing of monoclonal antibodies and antibody recombinant production
[0048] The heavy chain variable region gene fragment was digested with restriction endonucleases EcoRI and NheI, the light chain variable region gene fragment was digested with restriction endonucleases AgeI and BsiWl, and then purified by DNA purification kit , and digested with the same restriction endonuclease heavy chain expression vector pFUSEss-CHIg-hG1 and light chain expression vector pFUSE2ss-CLIg-hk (invivogen company). The heavy chain and light chain ligation products were transformed into competent cells DH5α Escherichia coli, and spread on Zeo+ plates and Blas+LB plates, respectively. The obtained positive clones were sent to Nanjing Qingke Company for sequencing. Finally, unique V-region nucleotide / protein sequences of NJDT001 and NJDT002 were obtained.
[0049] Sequence information:
[0050] NJDT001 heavy chain variable region amino acid sequence: as sh...
Embodiment 3
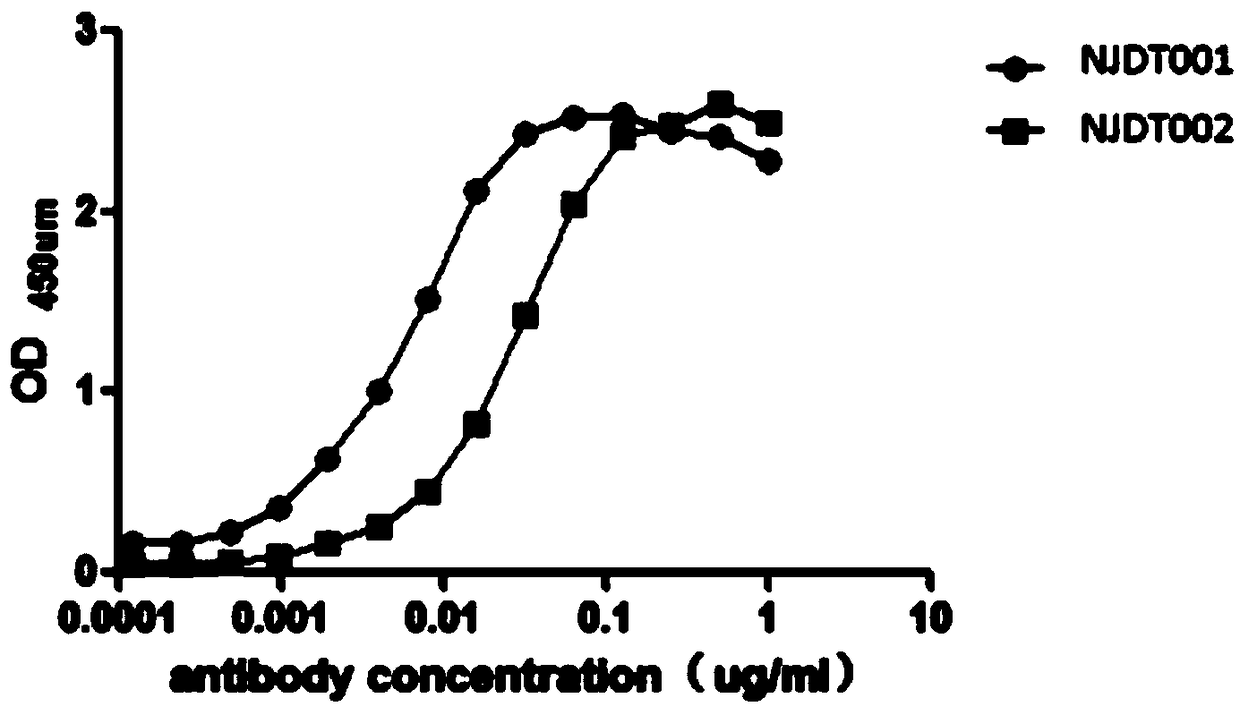
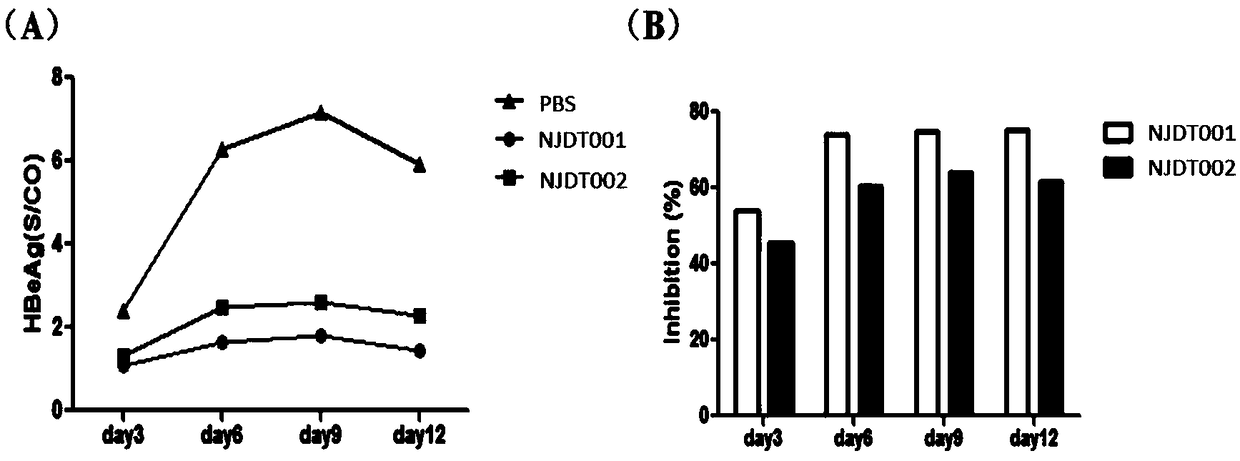
[0067] Embodiment 3: the binding of monoclonal antibody to surface antigen recombinant protein
[0068] Equally dilute the purified monoclonal antibody with PBS, add 50ul of antibody solution of corresponding concentration in the sample well according to the concentration from high to low, and set up 3 duplicate wells, add 50ul of negative and positive controls; Add 50ul of enzyme-labeled reagent to each well, except for blank wells, shake gently to mix; seal the plate with a sealing film, and incubate in a 37°C incubator for 30 minutes; carefully peel off the sealing film, and wash 5 times with a plate washer , as far as possible for the last time; add 50u of developer A to each well, then add 50ul of developer B, shake gently to mix, and develop color at 37°C in the dark for 15 minutes; add 50ul of stop solution to each well, shake gently Mix well and measure the result within 10 minutes. Set the dual wavelength of the microplate reader to 450nm / 610nm, and measure the OD va...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap