Preparation and application of PD-L1-targeted polypeptide derivative and 99mTc complex thereof
A technology of PD-L1 and peptide derivatives, which is applied in the field of preparation and application of PD-L1-targeting peptide derivatives and their 99mTc complexes, can solve the problems of not reflecting the immune status of tumors, and achieve a simple and stable labeling method Good performance and high marking rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
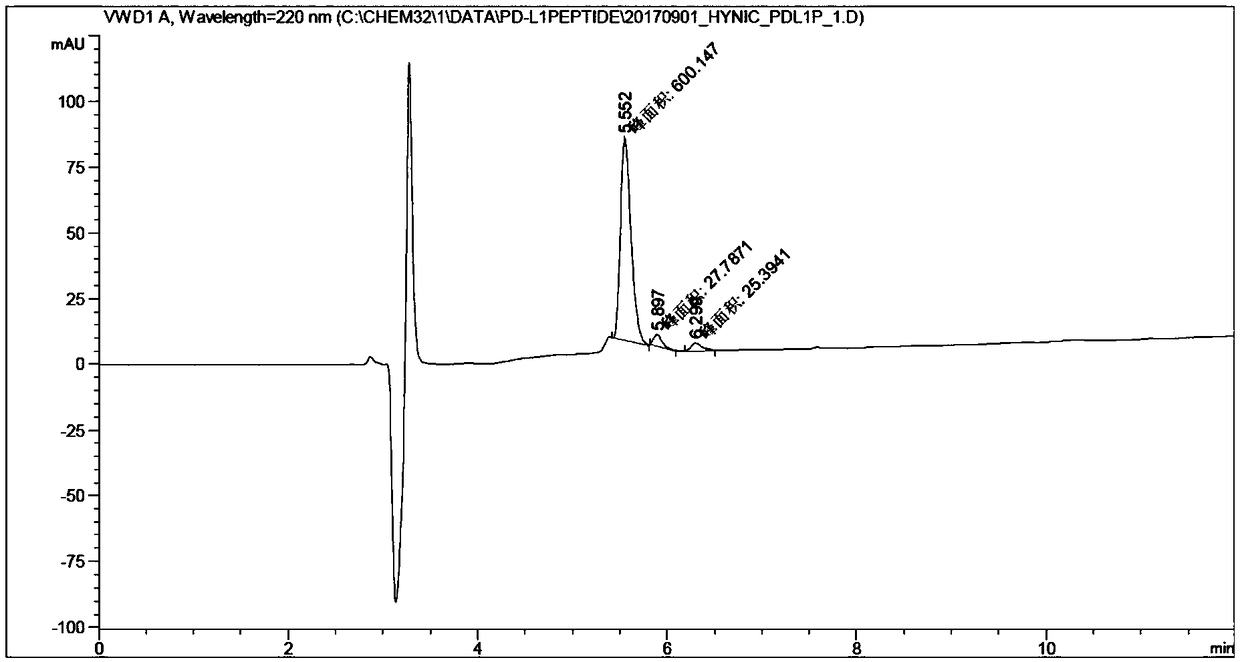
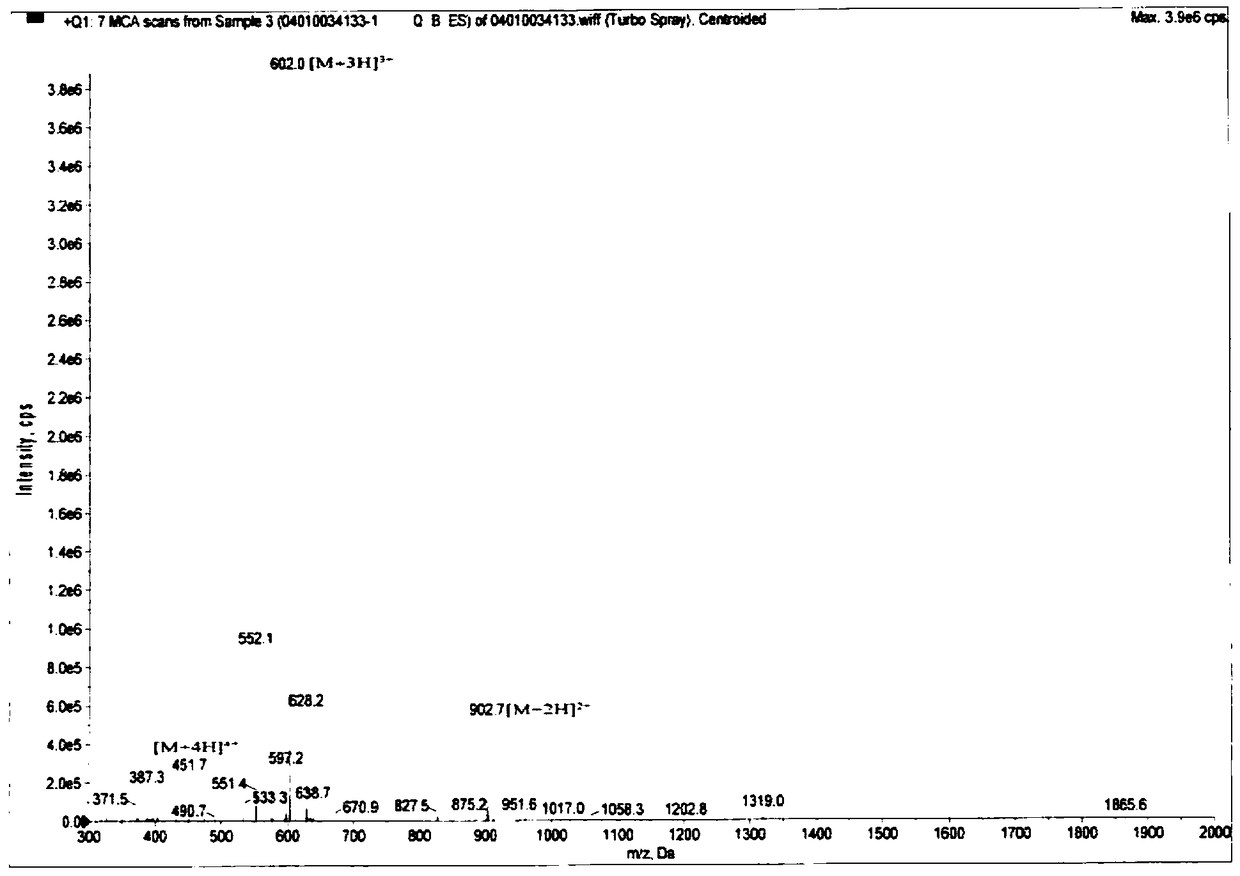
[0029] The synthesis of embodiment 1 polypeptide compound HYNIC-Acp-NYSKPTDRQYHF
[0030] It was synthesized by solid phase synthesis. Take Wang-Phe-NH 2 Resin is the starting material, add 3 times molar equivalents of Fmoc-His-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Arg-OH, Fmoc-Asp(tBu) in sequence -OH, Fmoc-Thr(tBu)-OH, Fmoc-Pro-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Asn(Trt )-OH, Fmoc-Acp-OH and Boc-Hynic. Each amidation reaction uses 6-fold molar equivalents of HBTU and 6-fold molar equivalents of N,N-diisopropylethylamine as catalysts. After each amidation reaction, the Fmoc protecting group was removed with 20% piperidine, and finally trifluoroacetic acid was used to remove the remaining protecting group and Wang-Phe-NH 2 resin. The polypeptide was isolated and purified by semi-preparative HPLC. The product was characterized by mass spectrometry and HPLC. Its spectrogram is attached figure 1 and 2 .
Embodiment 2L-99
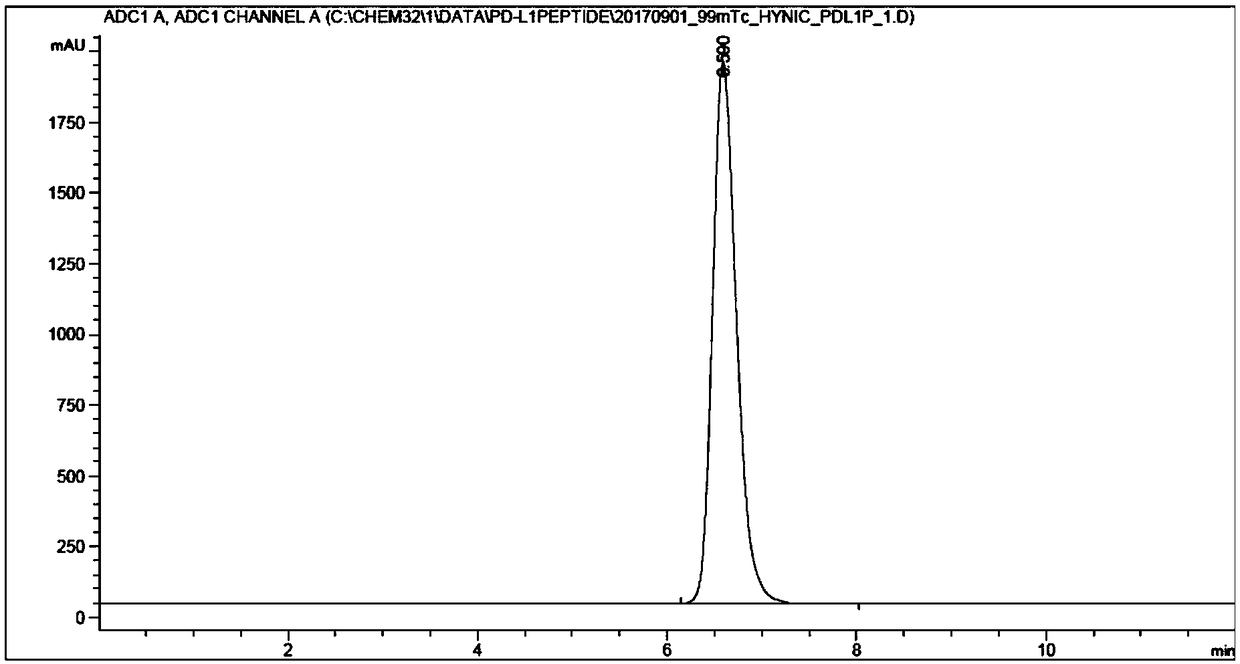
[0031] Example 2L- 99mSynthesis of Tc-HYNIC-Acp-NYSKPTDRQYHF Complex
[0032] Take 10 μg of the above product HYNIC-Acp-NYSKPTDRQYHF and add it to the mixture of 0.5mL EDDA solution (20mg / mL dissolved in 0.1M NaOH solution) and 0.5mL Tricine solution (40mg / mL dissolved in 0.05M pH 6.0 phosphate buffer) Dissolve, then add 25 μL SnCl 2 solution (0.1-10mg / mL dissolved in 0.01-1M HCl), 30mCi Na 99m TCO 4 Solution, react at 100°C for 10min. After the reaction, the reaction solution was sterilized by a 0.22 μm Millipore needle filter. The product does not need to be purified, and the labeling rate is >99%.
[0033]
[0034] The above-mentioned labeled products were identified by reversed-phase high-performance liquid chromatography.
[0035] Reversed-phase high-performance liquid chromatography method: the chromatographic column is Agilent ZOBRAX C-18 column (5 μm, 4.6×250 mm). The mobile phase was acetonitrile / 0.1% trifluoroacetic acid / water. The washing conditions are 0...
Embodiment 3
[0037] Embodiment 3 polypeptide compound HYNIC-NH 2 -(CH 2 ) m Synthesis of -C(O)-NYSKPTDRQYHF
[0038] It was synthesized by solid phase synthesis. Take Wang-Phe-NH 2 The resin is the starting material, and Fmoc-His-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Gln(Trt)-OH, Fmoc-Arg-OH, Fmoc-Asp( tBu)-OH, Fmoc-Thr(tBu)-OH, Fmoc-Pro-OH, Fmoc-Lys(Boc)-OH, Fmoc-Ser(tBu)-OH, Fmoc-Tyr(tBu)-OH, Fmoc-Asn (Trt)-OH, Fmoc-NH 2 -(CH 2 ) m -COOH (m=1-20) and Boc-Hynic. Each amidation reaction uses 1-6 molar equivalents of HBTU and 1-10 molar equivalents of N,N-diisopropylethylamine as catalysts. After each step of the amidation reaction, the Fmoc protecting group was removed with 5%-50% piperidine, and finally trifluoroacetic acid was used to remove the remaining protecting group and Wang-Phe-NH 2 resin. The polypeptide was isolated and purified by semi-preparative HPLC.
PUM
| Property | Measurement | Unit |
|---|---|---|
| radioactivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com