Bacterially derived, intact minicells for delivery of therapeutic agents to brain tumors
A technology of microcells and brain tumors, which can be used in preparations for in vivo experiments, capsule delivery, anti-tumor drugs, etc., and can solve the problems of poor cure rate of brain cancer treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0202] Example 1. Preparation of doxorubicin-coated minicells targeting canine EGFR
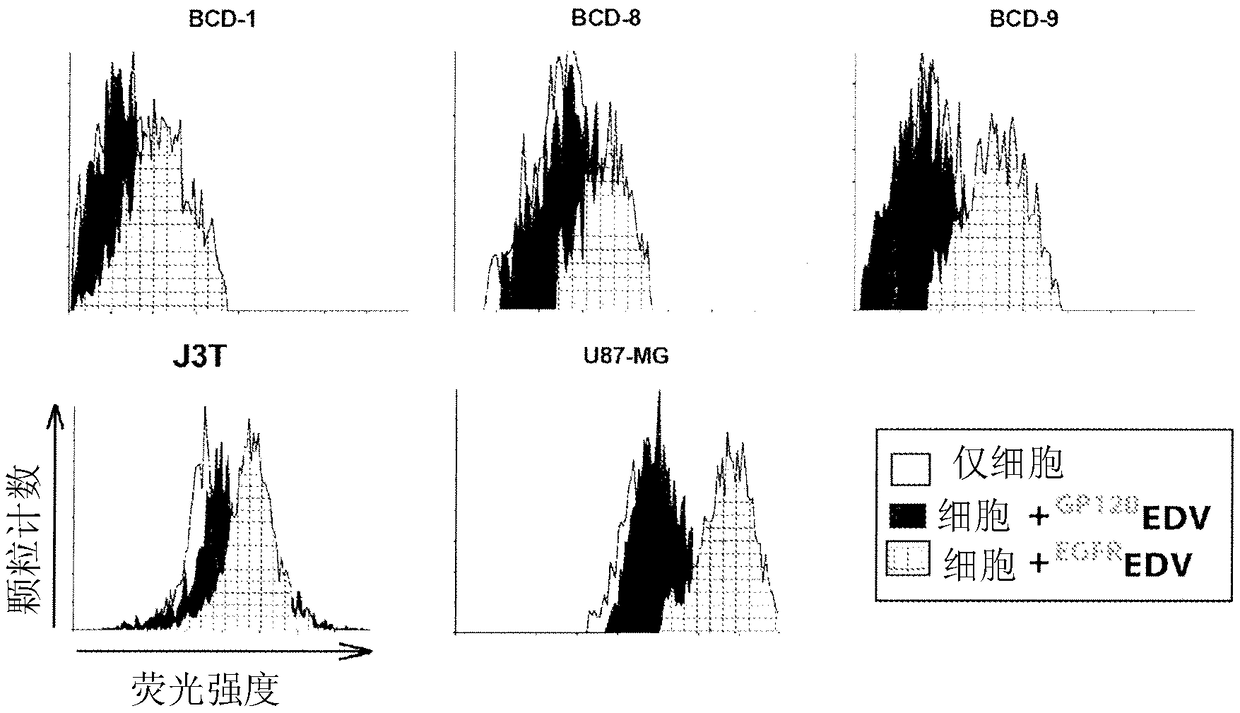
[0203] Minicells were derived from Salmonella typhi, a minCDE-chromosomal deletion mutant of S. typhimurium, purified, coated with doxorubicin (dox) and treated with bispecifics including anti-minicell surface O-glycan and anti-canine EGFR specificities Linked targeting of monoclonal antibodies (MAbs), (named EGFR microcell 阿霉素 ), as previously described by MacDiarmid et al. (2007).
[0204] Characterize first EGFR microcell 阿霉素 Their suitability for intravenous administration to seven dogs with advanced brain cancer (dogs designated BCD-1 to BCD-7). Two additional dogs, BCD-8 and BCD-9, presented to the veterinary care center but were not studied because of more advanced brain tumors and were euthanized. Brain biopsy samples provided respective brain tumor cells for in vitro studies.
Embodiment 2
[0205] Example 2. Characterization of anti-human EGFR monoclonal antibodies that bind to canine brain tumor cells
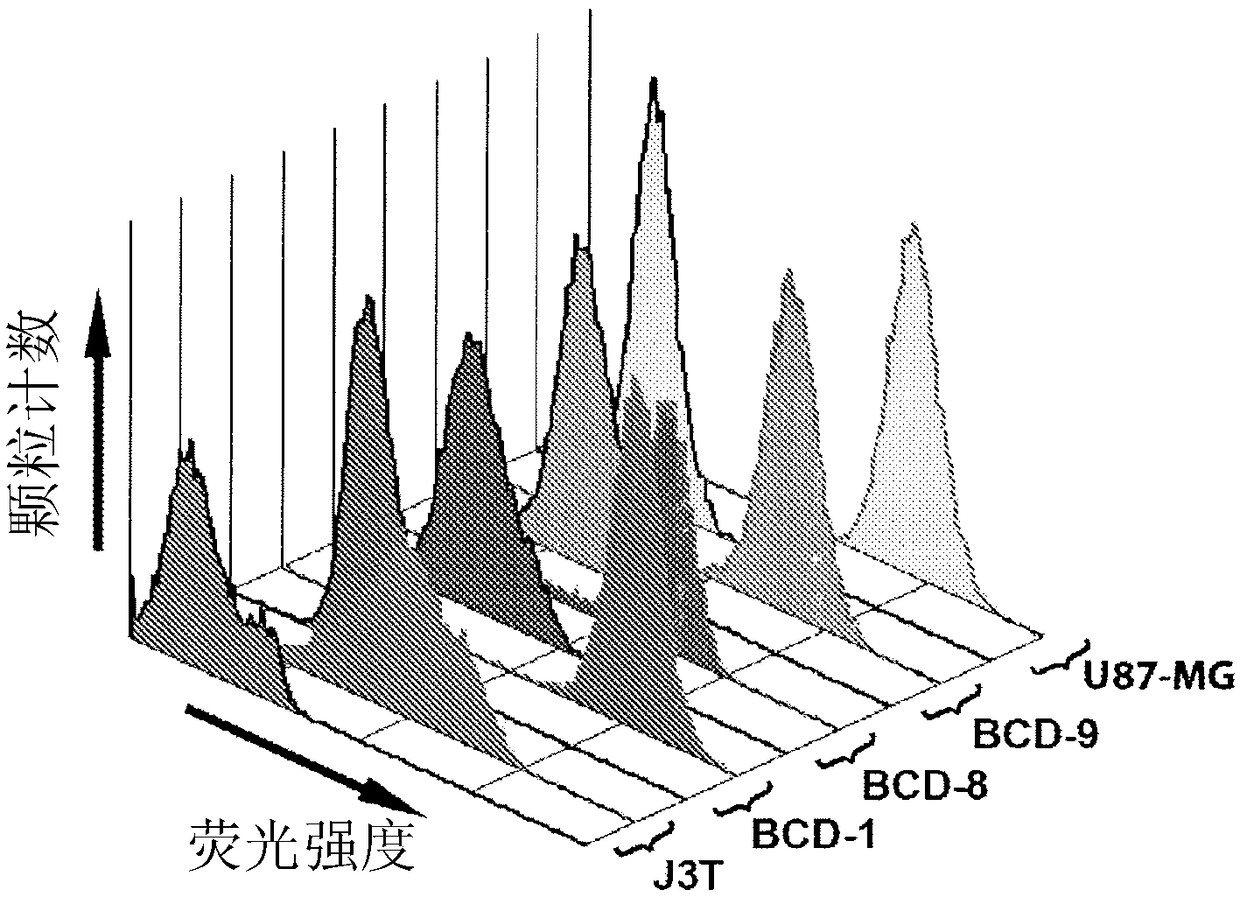
[0206] Upregulation and overexpression of EGFR is well known in -60% of GBM cases in humans (Smith et al., 2001) and dogs (Higgins et al., 2010). Given the non-availability of specific canine EGFR monoclonal antibodies, commercially available anti-human EGFR MAbs were tested in canine and human brain tumor cell lines to determine the cross-reactivity of the MAbs to EGFR on canine brain tumor cells.
[0207] Where feasible, brain tumor biopsy samples were obtained from case study dogs. Tissue samples from BCD-1, -8 and -9 were treated with 1 mg / ml in Dulbecco's modified Eagle's medium (DMEM) medium containing 10% fetal calf serum (FCS) and streptomycin Collagenase treatment for 10 min. Undigested tissue was removed by filtration through double sheets of sterile gauze. Collagenase digestion was stopped by diluting the cells with 5 ml of medium and centrifuging a...
Embodiment 3
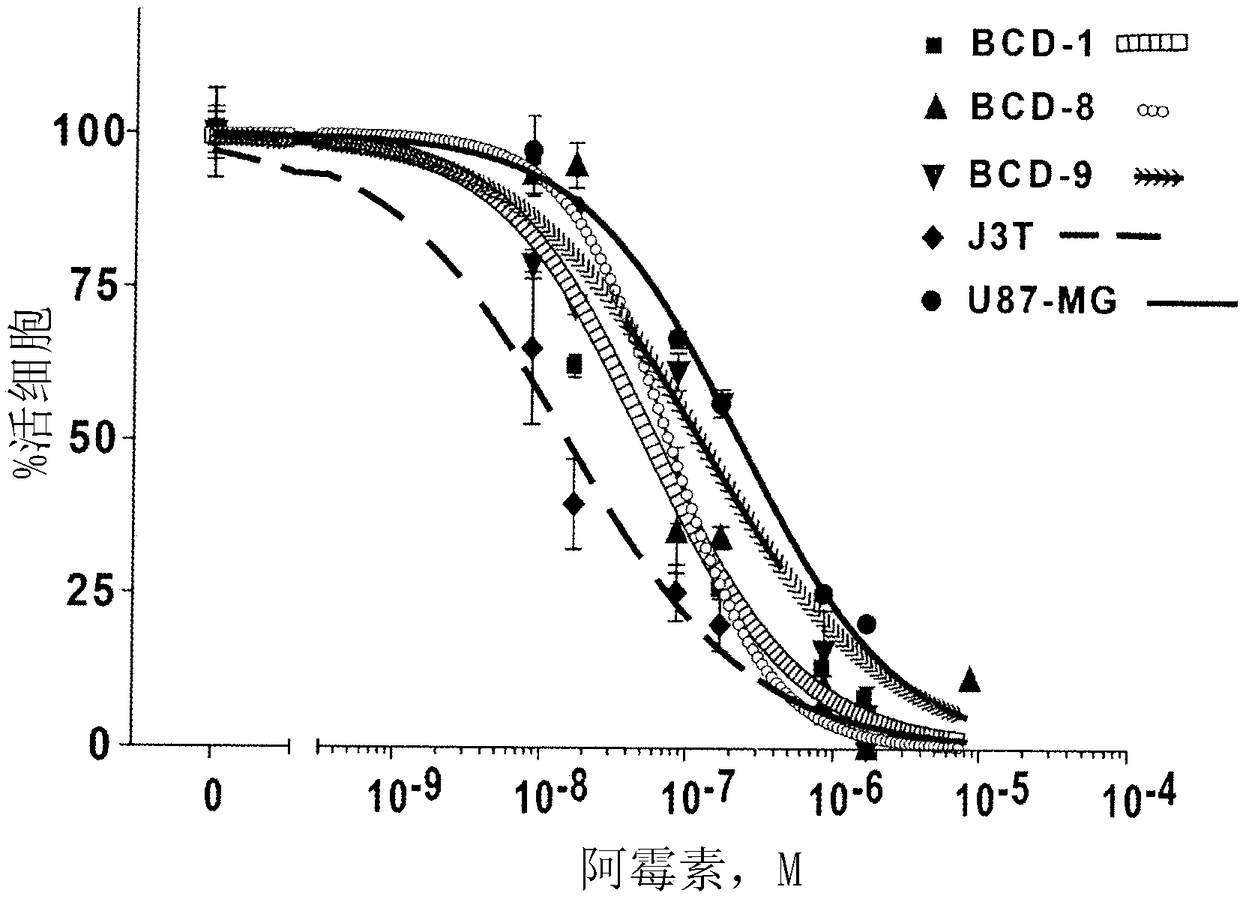
[0216] Example 3. Determination of the sensitivity of canine brain cancer cells to the chemotherapeutic drug doxorubicin
[0217] Before treating dogs with advanced brain cancer with doxorubicin-encapsulated, EGFR-targeted minicells, it is important to determine whether canine brain tumor cells are sensitive or resistant to the chemotherapeutic drug doxorubicin.
[0218] Canine brain tumor cells BCD-1, -8, -9 and J3T and human brain tumor cell line U87-MG were inoculated in a 96-well plate, 5x 10 per well 3 cells. at 37°C, 5% CO 2 The next cells were incubated overnight.
[0219] Doxorubicin was added to the cells in 100 μL of relevant media containing serum concentrations ranging from 1.7 nM to 8,600 nM and incubated for 72 hours.
[0220] To measure the cytotoxic effect of doxorubicin, an MTS cell proliferation assay was performed. 20uL of MTS solution (CellTitre Aqueous One MTS reagent - Promega) was added to each well and incubated for 30 minutes in the dark. Absorb...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com