Maternal-fetal blood group incompatibility treatment device based on clearance of pathogenic antibodies
A treatment device and a technology for incompatibility of blood types, applied in the medical field, can solve the problem of not raising Rh-positive "O" type red blood cells, etc., and achieve the effect of saving blood source and increasing fusion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] figure 1 It is a hybrid cell clone diagram prepared by the present invention.
[0016] figure 2It is the immunoagglutination diagram of the hybrid cell and Rh antibody prepared in the present invention.
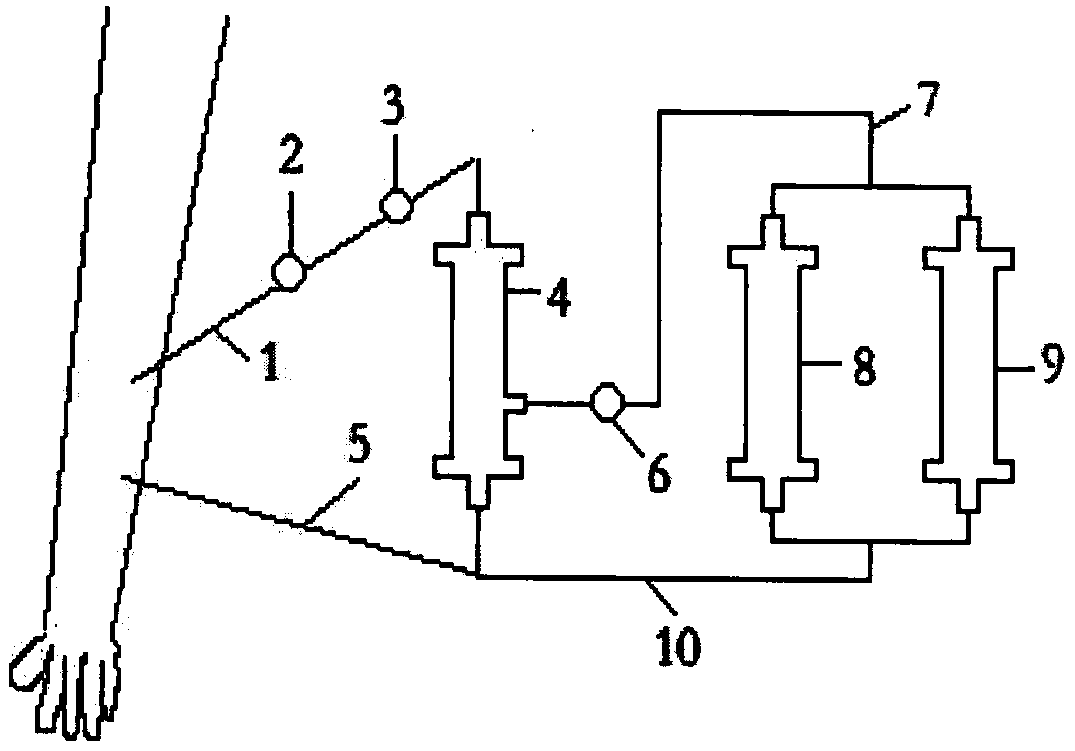
[0017] image 3 It is a schematic diagram of the treatment device for maternal-fetal blood group incompatibility based on hybrid strains to eliminate pathogenic antibodies of the present invention.
[0018] Figure 4 It is a schematic diagram of the internal structure of the plasma separator proposed according to the present invention.
[0019] Figure 5 It is a schematic diagram of the internal structure of the adsorber proposed according to the present invention
[0020] exist figure 1 Among them, Rh positive "O" type bone marrow cells are differentiated by erythropoietin-directed induction and immature red blood cell line. Under the action of PEG, after fusion with myeloma cells, they are screened by HAT for 1 to 2 weeks and viewed under an inverted microsco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com