Preparation of quality control reference strain for myeloid leukemia fusion gene detection
A fusion gene and leukemia technology, applied in the medical field, can solve the problems of affecting the detection method, unable to repeat, unable to transform bone marrow cells, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
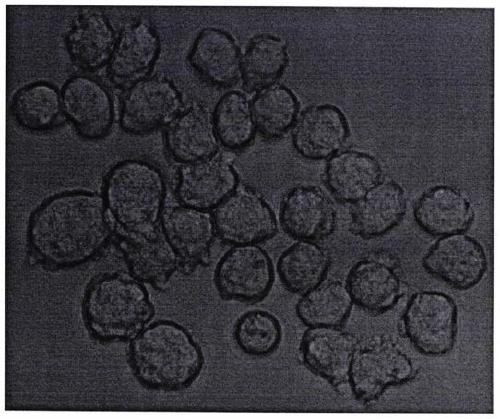
[0013] figure 1 It is a hybrid cell clone diagram prepared by the present invention.
[0014] exist figure 1 In the medium, the bone marrow cells of myeloid leukemia patients were induced by granulocyte colony-stimulating factor and differentiated granulocytes at various stages. Under the action of PEG, after fusion with mouse myeloma cells, they were screened by HAT for 1 to 2 weeks, and observed under an inverted microscope. Shoot (40X) to obtain a hybrid cell clone map.
[0015] Combine below figure 1 , the embodiment of the present invention is described in detail.
[0016] 1. Cells to be fused
[0017] (1) Bone marrow cells to be fused: Bone marrow cells from patients with myeloid leukemia who were diagnosed and left after bone marrow cytology examination contained BCR-ABL, PML-RARAα, AML1 / ETO and / or PML-RARa fusion genes.
[0018] (2) Myeloma cells: mouse SP2 / 0 tumor cells, purchased from Sigma Company.
[0019] 2. Experimental reagents
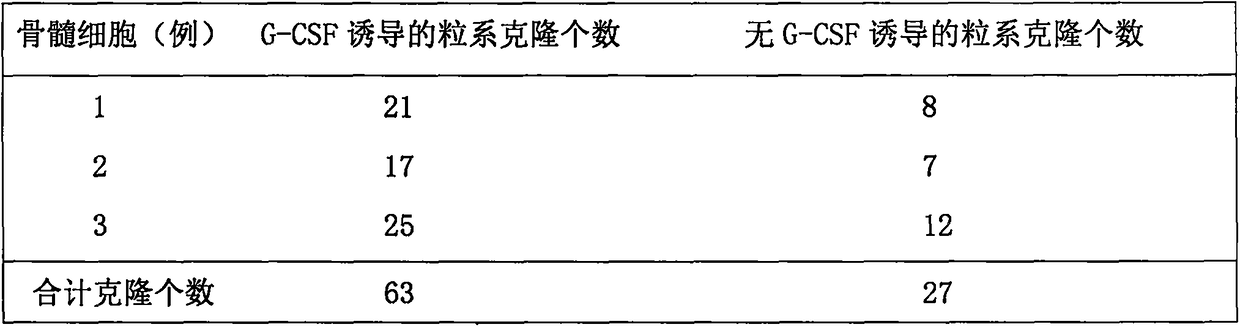
[0020] (1) Granulocyte co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com