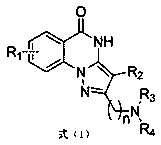
Pyrazoloquinazolone derivatives as parp inhibitors and uses thereof
A technology of pyrazoloquinazolinone and derivatives, which is applied in the field of pyrazoloquinazolinone derivatives, can solve the problems of no standard treatment plan, limited drug delivery efficiency, difficult brain tumor onset, etc., and achieves oral administration. Good bioabsorption, good application prospect, significant selective inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1: The synthesis of compound I-1 is prepared according to general scheme 1, and the specific method is as follows:
[0050]
[0051]Step 1 Preparation of SMb1: Add diethyl oxalate d1 (5 g) to a solution of sodium ethoxide (2.55 g, 37.7 mmol) in ethanol (50 ml), stir at 60°C for 0.5 h, and dissolve propionitrile e1 (3 ml) Added to the mixture and heated to reflux for 7 h. The reactant was cooled to room temperature, and the solid was collected by filtration, washed with ether, and dried naturally to obtain SMb1, which was directly used in the next reaction.
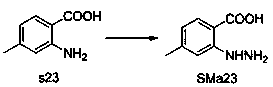
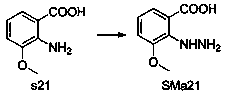
[0052] Step 2 to prepare c1: It can be prepared by general method A and general method B.
[0053] General method A: The raw material SMa1 is dissolved in acetic acid, SMb1 is added, the mixture is reacted in a microwave reactor at 150° C. for 5-10 minutes, the precipitate is collected by filtration, washed with ether, and recrystallized from ethyl acetate / ethanol to obtain c1.
[0054] General metho...
Embodiment 2
[0058] Example 2: Preparation 1-2.
[0059] Dissolve I-1 (15 mmol) and methyl iodide (15 mmol) in ethanol, add sodium ethoxide, stir at 0°C to 40°C for 6h-10h, recover the solvent through silica gel column chromatography (ethyl acetate: petroleum ether 1 :3) I-2 was isolated. MS m / z (ESI): 243.1[M+1] + . 1 HNMR (400 MHz, DMSO-d6) δ 11.31 (s, 1H), 10.24 (s, 1H), 7.50-7.52 (m,1H), 7.81 (dd, 1H), 8.04 (d,1H) , 8.09 (dd , 1H), 4.18 (s,2H), 3.23 (s,3H), 2.05 (s,3H ).
Embodiment 3
[0060] Example 3: Preparation 1-3.
[0061] With the method of Example 2, dissolve I-1 (10 mmol) and methyl iodide (30 mmol) in ethanol, add sodium ethylate, reflux and stir for 18 h-24 h, recover the solvent through silica gel column chromatography (ethyl acetate : Petroleum ether 1:5) to obtain I-3. MS m / z(ESI): 257.1 [M+1] + . 1 HNMR (400 MHz, DMSO-d6) δ 11.46 (s, 1H), 7.51-7.55 (m,1H), 7.86 (dd, 1H), 8.03 (d,1H), 8.14 (dd, 1H), 3.65 (s ,2H), 2.06 (s,3H ), 2.18 (s,6H ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com