A tumor suppressive antibody targeting PD-1 and its application
A PD-1 and antibody technology, applied in the medical field, can solve problems such as inability to effectively stimulate T cell immunity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
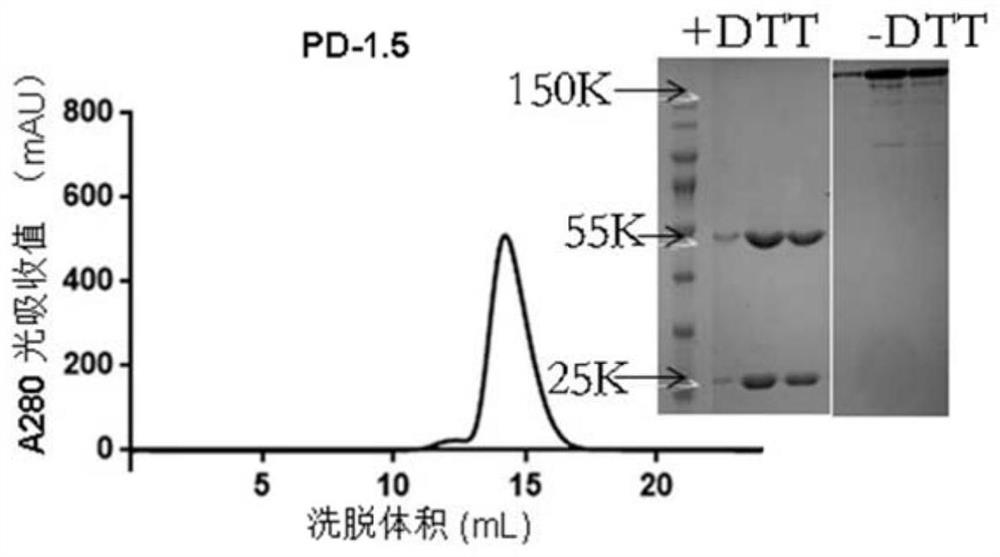
[0052] Example 1. Preparation of PD-1.5 Antibody
[0053] 1. Construction of PD-1 recombinant expression plasmid
[0054] Using the cDNA of PD-1 (NM_005018.2) as a reference, the DNA sequence of PD-1 (amino acid 1-170) was synthesized, and restriction sites EcoRI and BglII were respectively introduced, and six His amino acid tags were introduced at the C-terminal, where The EcoRI restriction site is located at the 5' end of the sequence, and the 6-His amino acid tag and the restriction site BglII are located at the 3' end of the sequence in turn. The DNA sequence of the synthesized PD-1 was cloned into the expression vector pCAGGS (Addgene) by using the restriction sites EcoRI and BglII to establish a recombinant eukaryotic expression plasmid for the full-length PD-1 protein.
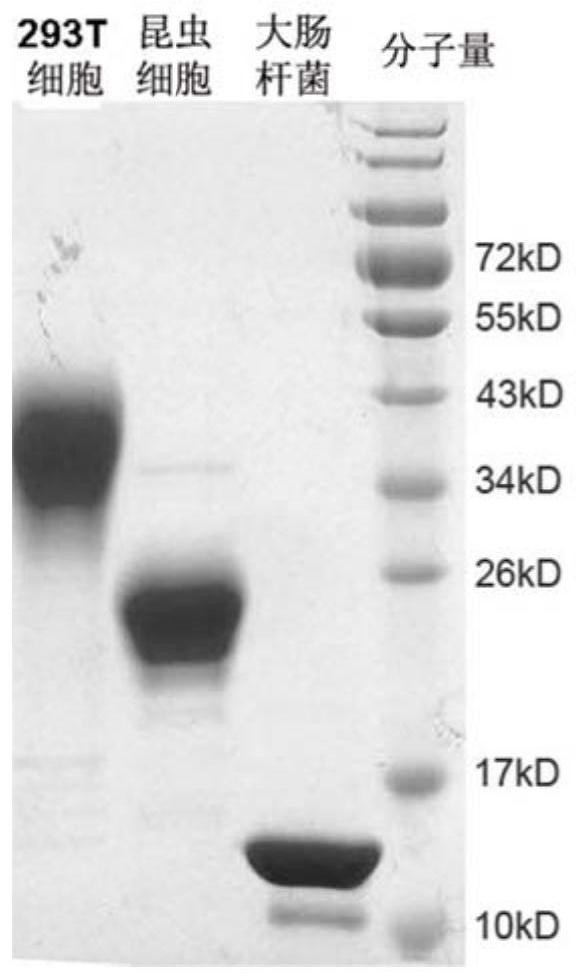
[0055] 2. Expression and purification of PD-1 recombinant protein
[0056] 1) Transfection of HEK293T cells: HEK293T cells (ATCC) were transferred to culture dishes at a ratio of 1:3; 7.5mL of DMEM (w...
Embodiment 2
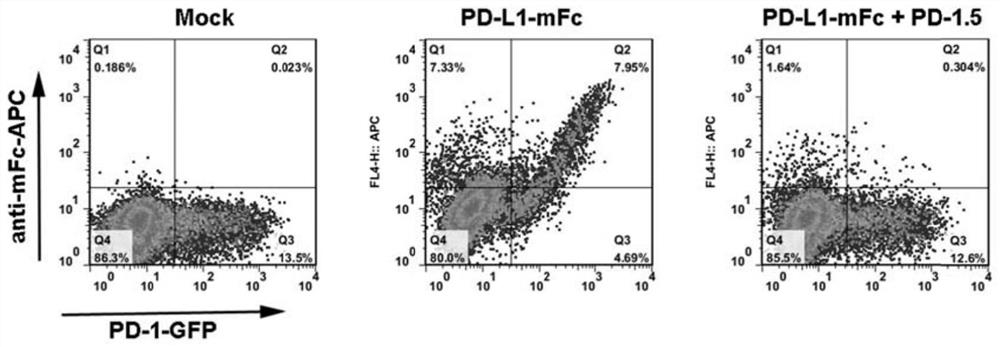
[0074] Example 2. PD-1 blocking antibody screening and affinity analysis
[0075] Mice were immunized with the PD-1 protein expressed in vitro by 293T cells, and the obtained monoclonal antibodies were screened for antibodies that could specifically block the interaction between PD-1 and PD-L1 by blocking experiments on PD-1 and PD-L1.
[0076] 1. Preparation of 293T cells expressing full-length PD-L1
[0077] In this example, 293T cells (ATCC) expressing PD-L1 were obtained by transfecting the PD-L1-GFP-p plasmid (Clontech) containing the full length of PD-L1 (pEGFP-N1 vector-GFP tag plasmid) into 293T cells (ATCC). Full-length 293T cells. 1 day before transfection according to 0.5~2×10 5 The cells were inoculated into each well of a 24-well culture plate, and 500 μl of complete DMEM medium (GIBCO Company) without antibiotics was added to ensure that the cells reached 70-80% confluence during transfection. 1 μg of PD-L1-GFP-p plasmid was diluted in 50 μL medium without ser...
Embodiment 3
[0080] Example 3. In vitro activation ability of PD-1 blocking antibody on tumor-specific T cells in non-small cell lung cancer cases
[0081] One of the important applications of PD-1 blocking antibody is its anti-tumor effect. In this example, the peripheral blood of 15 cases of non-small cell lung cancer was collected, and tumor-specific polypeptides were used for in vitro culture, and then the effect of PD-1.5 antibody on tumor polypeptides was evaluated. The activation ability of library-specific T cells is detected to evaluate the anti-tumor potential of the PD-1 blocking antibody screened by the present invention at the cellular level in vitro.
[0082] 1. Case enrollment screening
[0083] The cases included in this example were non-small cell lung cancer cases with positive tumor cells in needle biopsy.
[0084] 2. PBMCs sorting
[0085] The lymphocytes used in the present invention are obtained from the venous peripheral blood of an individual. After the selected ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com