A kind of nano antibody against rabies virus g protein and its application
A nanobody, rabies virus technology, applied in antiviral immunoglobulins, antiviral agents, viral antigen components, etc., can solve the problems of lack of CH1 in heavy chain, lack of light chain binding ability, inability to express heavy chain CH1 domain, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1. Preparation of rabies virus G protein
[0044] 1.1 Transfer vector construction
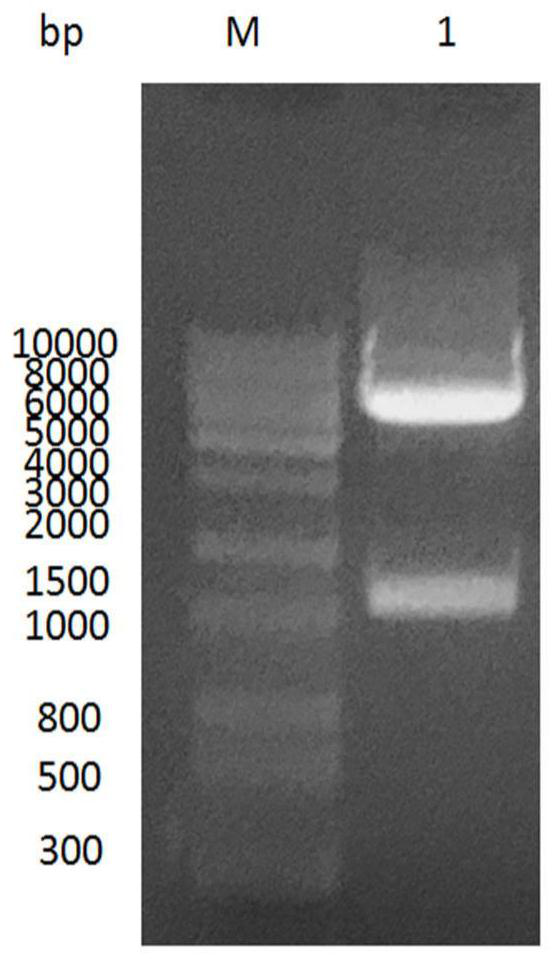
[0045] The rabies virus G protein extramembrane region (AAV85905.1) plasmid PUC57-RVG (synthesized by Jinweizhi Company, containing a His tag at the C-terminus) and the carrier PFastBac HTB (purchased from Biofeng Company) were double-introduced by BamⅠ and PstⅠ simultaneously. Digest with Dicer, recover the G protein extramembrane region gene (1.4kb) and the linearized target vector fragment (4.8kb), use T 4 Ligase ligation, named PFastBac HTB-RVG, transformed into DH5α host bacteria, screened for double resistance to gentamicin and ampicillin, selected positive colonies, amplified and extracted plasmids, and identified by double enzyme digestion with BamⅠ and PstⅠ and sequenced ( figure 1 : M is Trans 2K DNAmarker; 1 is the product of PFastBac HTB-RVG digested by BamI and PstI).
[0046] 1.2 Construction of expression plasmid
[0047] After overnight amplification and e...
Embodiment 2
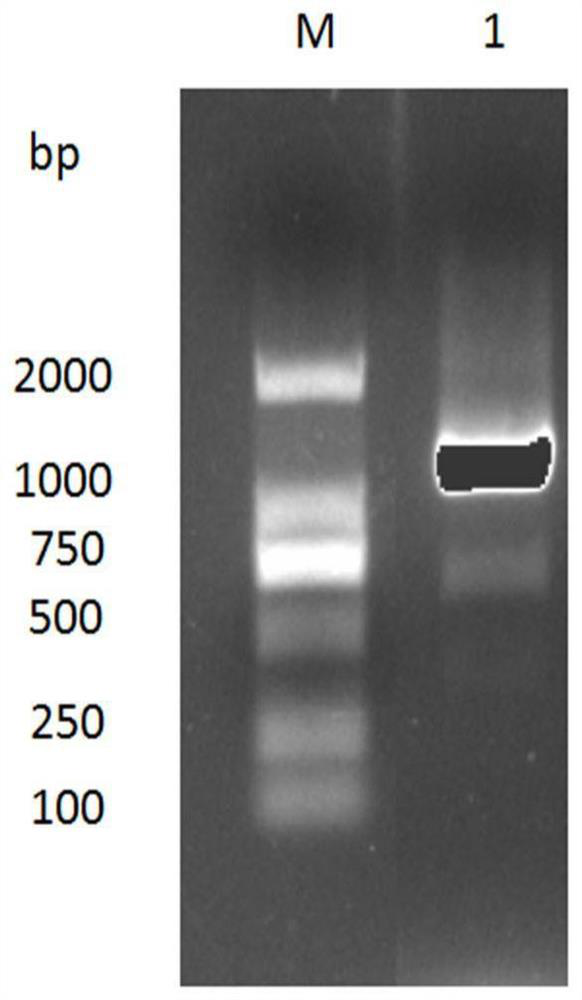
[0053] Example 2. Construction and screening of anti-rabies virus G protein nanobody phage display library
[0054] 2.1 Immunity of alpacas
[0055] Select a healthy adult alpaca, mix the purified rabies virus G protein with Freund's adjuvant at a ratio of 1:1, and immunize the alpaca with 6-7μg / kg subcutaneous injection at multiple points on the back. times, the immunization interval was 2 weeks. Afterwards, the peripheral blood of the alpaca was collected for the construction of a phage display library.
[0056] 2.2. Isolation of camel-derived lymphocytes
[0057] Lymphocytes were separated from the collected camel-derived anticoagulated whole blood according to routine procedures in this technical field, and each 2.5×10 7 Add 1mL RNA isolation reagent to each living cell, take 1mL for RNA extraction, and store the rest at -80°C.
[0058] 2.3 Total RNA extraction
[0059] Total RNA was extracted according to routine procedures in the technical field, and the concentrati...
Embodiment 3
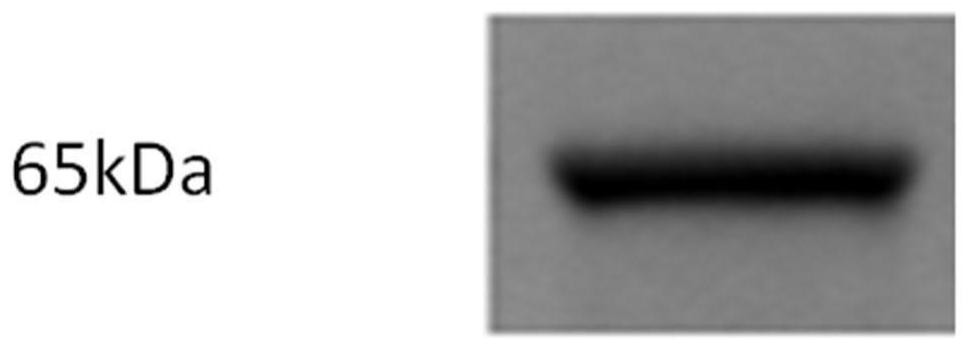
[0092] Example 3. Affinity activity determination of anti-rabies virus G protein nanobody and antigen
[0093] 3.1 Chip antigen coupling: Prepare the antigen with different pH sodium acetate buffer (pH5.5, pH5.0, pH4.5, pH4.0) to make 20μg / mL working solution, and prepare 50mM NaOH regeneration solution at the same time, The template method in the instrument of Biacore T100 protein interaction analysis system was used to analyze the electrostatic binding between the antigens at different pH conditions and the surface of the chip (GE Company). Use a neutral pH system and adjust the antigen concentration as required for coupling conditions. The chip was coupled according to the template method included in the instrument: select the blank coupling mode for channel 1, select the Target coupling mode for channel 2, and set the target to the designed theoretical coupling amount. The coupling process takes about 60 min.
[0094]3.2 Exploration of analyte concentration setting condi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com