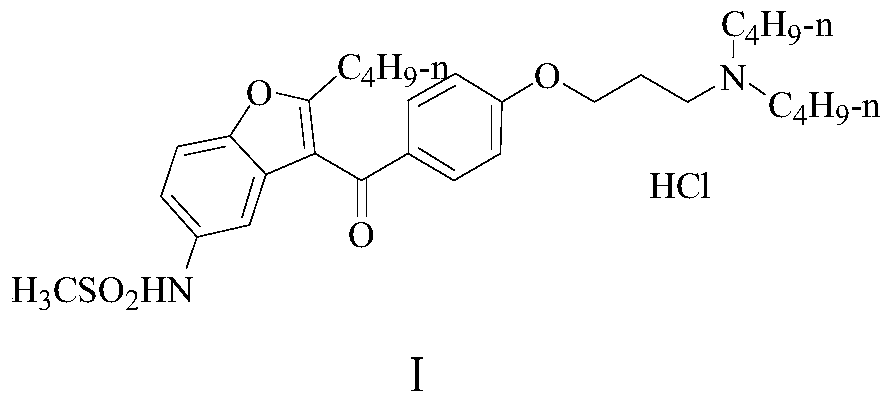
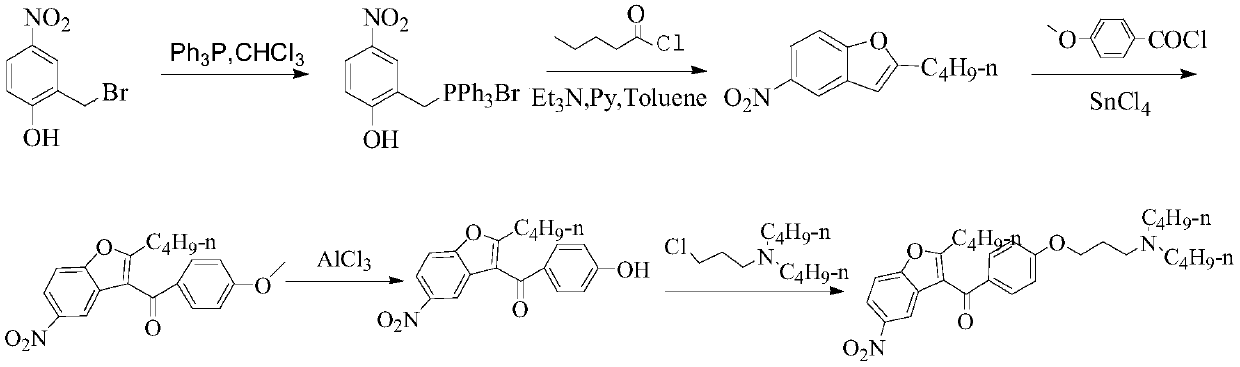
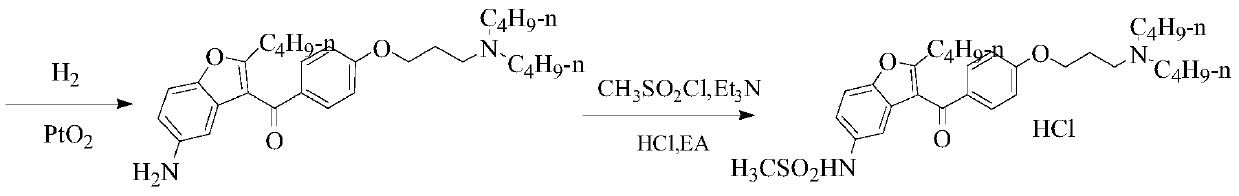A kind of preparation method of dronedarone hydrochloride
A technology of dronedarone hydrochloride and hydrochloric acid, applied in the field of pharmaceutical synthesis, can solve problems such as difficulty in large-scale industrial production, increase in production cost, etc., and achieve the effects of less product impurities, high liquid phase purity, and fewer operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] A preparation method of dronedarone hydrochloride (I), comprising the following steps:
[0072] Add 1500 g of 1,2-dichloroethane, 112.0 g (1.0 mol) of 1,4-cyclohexanedione, and 3.0 g of 30% hydrochloric acid aqueous solution into a 5-liter four-neck flask, cool and keep the internal temperature at 10-15°C In between, 156.0 g (2.2 mol) of chlorine gas was slowly introduced, and the reaction was stirred at 15-25° C. for 3 h until the reaction was complete. Then nitrogen was bubbled for 2 hours to blow off the generated hydrogen chloride and excess chlorine. Thereafter, 390 grams (1.0 mol) of 1-[4-(3-dibutylamino-propoxy)-phenyl]-heptane-1,3-dione (VI) was added to the resulting reaction liquid, 152 gram of potassium carbonate, stirred and reacted at 55-60°C for 5 hours, cooled to 20°C, filtered, layered, added 30 grams of 10% hydrochloric acid aqueous solution to the gained organic layer, stirred and reacted at 40-50°C for 4 hours, divided layer, the aqueous layer was e...
Embodiment 2
[0078] A preparation method of dronedarone hydrochloride (I), comprising the following steps:
[0079] Add 1500 grams of 1,2-dichloroethane, 112.0 grams (1.0mol) of 1,4-cyclohexanedione, 5.0 grams of 40% hydrobromic acid aqueous solution to a 5-liter four-neck flask, cool, and keep the inner temperature at 10- 322.0 g (2.0 mol) of liquid bromine was slowly added dropwise at 15°C, and the reaction was stirred at 15-25°C for 2 hours until the reaction was complete. Then nitrogen gas was bubbled for 2 hours to blow off the generated hydrogen bromide gas. Thereafter, 390 grams (1.0 mol) of 1-[4-(3-dibutylamino-propoxy)-phenyl]-heptane-1,3-dione (VI) was added to the resulting reaction liquid, 152 gram of potassium carbonate, stirred and reacted at 55-60°C for 5 hours, cooled to 20°C, filtered, layered, added 30 grams of 10% hydrochloric acid aqueous solution to the gained organic layer, stirred and reacted at 40-50°C for 4 hours, divided layer, the aqueous layer was extracted tw...
Embodiment 3
[0081] A preparation method of dronedarone hydrochloride (I), comprising the following steps:
[0082]Add 1500 grams of 1,2-dichloroethane, 112.0 grams (1.0mol) of 1,4-cyclohexanedione, and 4.0 grams of anhydrous ferric chloride to a 5-liter four-neck flask, cool and keep the inner temperature at 10- Between 15°C, 156.0 g (2.2 mol) of chlorine gas was slowly introduced, and the reaction was stirred at 15-25°C for 5 hours until the reaction was complete. Then nitrogen was bubbled for 2 hours to blow off the generated hydrogen chloride and excess chlorine. Thereafter, 390 grams (1.0 mol) of 1-[4-(3-dibutylamino-propoxy)-phenyl]-heptane-1,3-dione (VI) was added to the resulting reaction liquid, 152 gram of potassium carbonate, stirred and reacted at 55-60°C for 5 hours, cooled to 20°C, filtered, layered, added 30 grams of 10% hydrochloric acid aqueous solution to the gained organic layer, stirred and reacted at 40-50°C for 4 hours, divided layer, the aqueous layer was extracted...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com