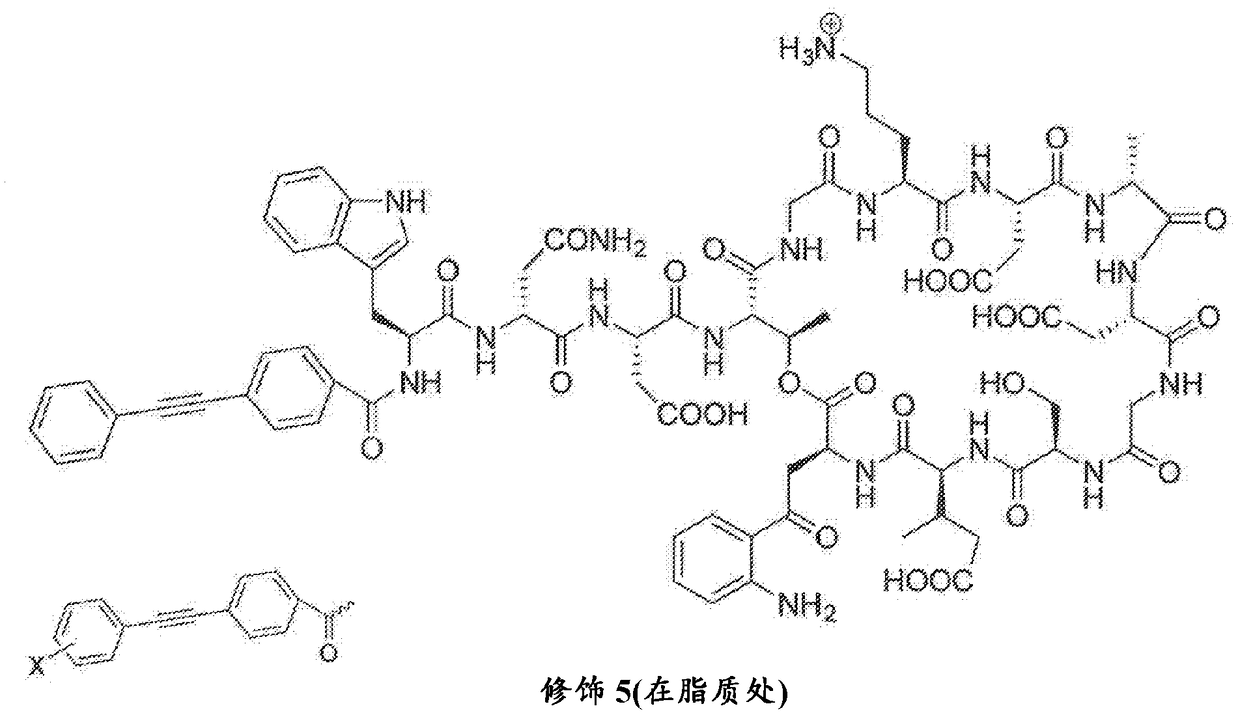
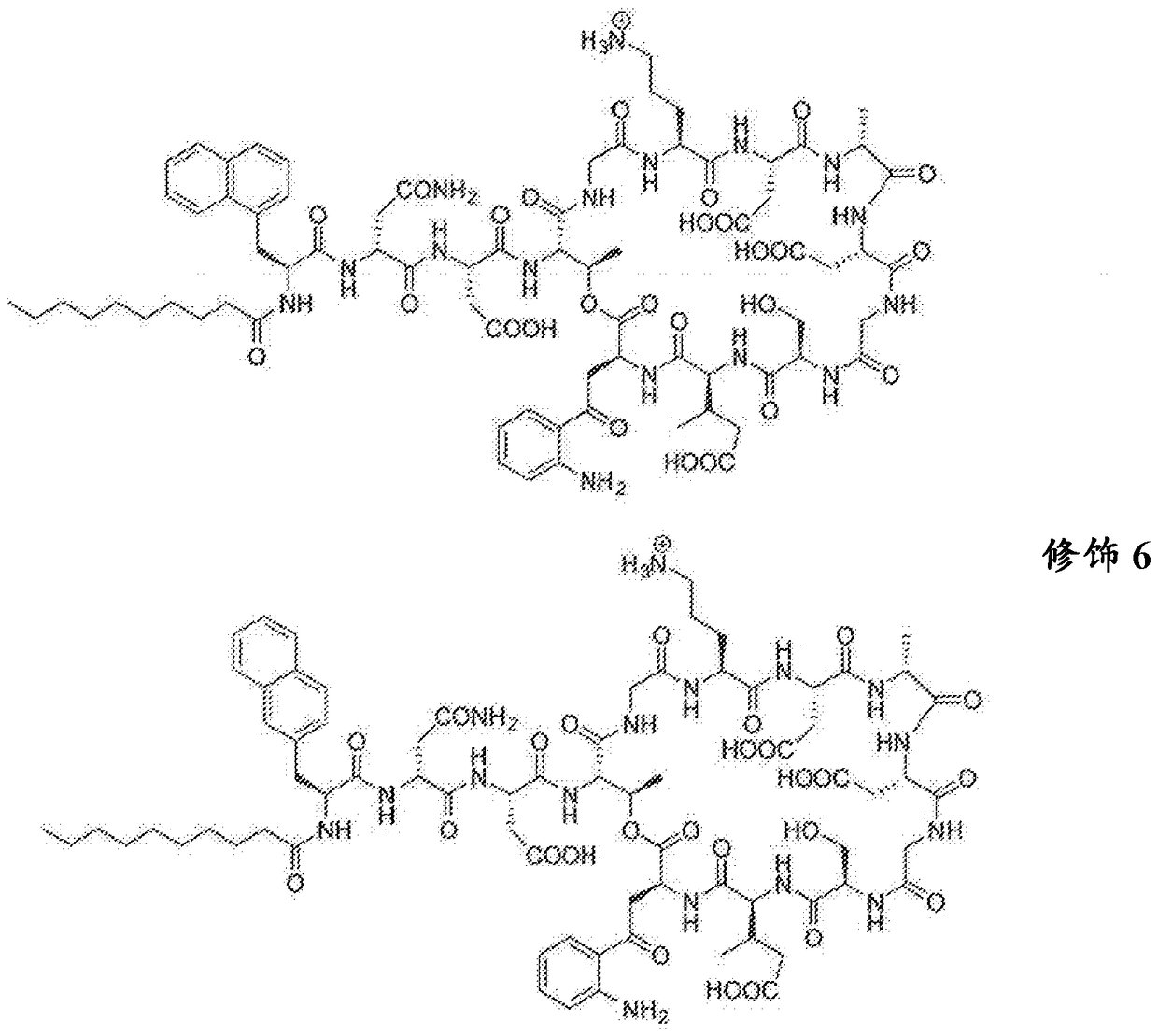
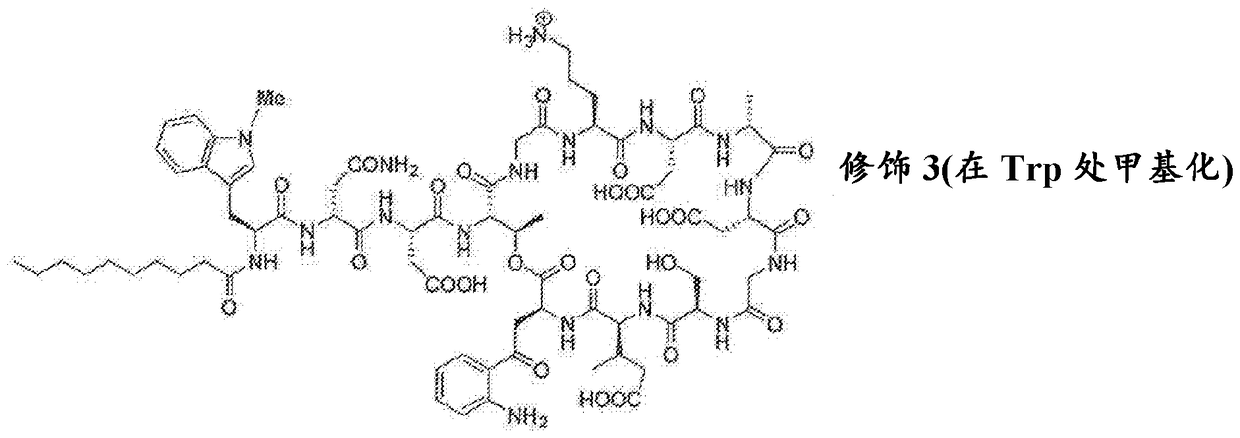
Antibacterial cyclic lipopeptides
A cyclolipopeptide and cycloalkynyl technology, applied in the field of antibacterial agents, can solve the problems of non-natural amino acid substitution of daptomycin and inability to generate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0235] Embodiment 1. Synthesis of compound 1
[0236] Synthesis of linear peptide resin-Gly-Asp(tBu)-DAla-Asp(tBu)-Orn(Boc)-Gly-Thr[O- meKyn-mGlu(tBu)-DSer(tBu)]-Asp(tBu)-DAsn(Trt)-Trp(Boc)-C 9 h 19 . The peptide was cleaved from 2-chlorotrityl resin under mild conditions (TFE / AcOH / DCM). After drying, the peptide was cyclized for an additional 4 hours using HATU / DCM. The solution was then concentrated and then treated with a cocktail containing 95% TFA and 2.5% water for 10 minutes. The crude product was purified by preparative RP-HPLC to afford the methylated Kyn-containing daptomycin analog Compound 1. [M+H] + Calculated 1635.7, [M+H] + The measured value is 1635.7, [M+2H] 2+ 818.1.
[0237]
Embodiment 2
[0238] Embodiment 2. Synthesis of compound 2
[0239] The linear peptide resin-Gly-Asp(tBu)-DAla-Asp(tBu)-Orn(Boc)-Gly-Thr[O- Kyn-mGlu(tBu)-DSer(tBu)]-Asp(tBu)-DAsn(Trt)-meTrp-C 9 h 19 . The peptide was cleaved from 2-chlorotrityl resin under mild conditions (TFE / AcOH / DCM). After drying, the peptide was cyclized for an additional 4 hours using HATU / DCM. The solution was then concentrated and then treated with a mixture containing 95% TFA and 2.5% water for 10 minutes. The crude product was purified by preparative RP-HPLC to yield the methylated TRP-containing daptomycin analog Compound 2. [M+H] + Calculated 1635.7, [M+H] + The measured value is 1635.6, [M+2H] 2+ 818.0.
[0240]
Embodiment 3
[0241] Embodiment 3. Synthesis of compound 3
[0242] Synthesis of linear peptide resin-Gly-Asp(tBu)-DAla-Asp(tBu)-Orn(Boc)-Gly-Thr[O- Kyn-mGlu(tBu)-DSer(tBu)]-Asp(tBu)-DAsn(Trt)-2Nal-C 9 h 19 . The peptide was cleaved from 2-chlorotrityl resin under mild conditions (TFE / AcOH / DCM). After drying, the peptide was cyclized for an additional 4 hours using HATU / DCM. The solution was then concentrated and then treated with a mixture containing 95% TFA and 2.5% water for 10 minutes. The crude product was purified by preparative RP-HPLC to give compound 3, a daptomycin analog containing 2-naphthyl Ala. [M+H] + Calculated value 1630.8, [M+H] + The measured value is 1630.8, [M+2H] 2+ 815.4.
[0243]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com