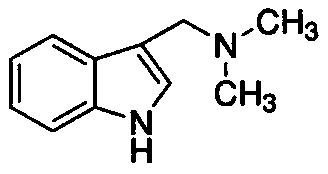
1-Methyl-5-bromophylline derivatives and their preparation methods and uses
A technology of rubarine and derivatives, which is applied in the field of 1-methyl-5-bromorubarine derivatives and preparation thereof, can solve problems such as high production cost, environmental pollution, low yield, etc., and achieves good resistance to plants. Effects of viral and bacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation method of 1-methyl-5-bromophylline derivative with chemical structural formula I is as follows:
[0035] The prepared 1-methyl-5-bromophylline derivative has the following chemical structure formula I:
[0036]
[0037] In the general formula I of the above chemical structure, HX represents an inorganic acid or an organic acid, and when HX represents an inorganic acid, it is HCl, HBr, HI, H 2 SO 4 or H 3 PO 4, when HX represents an organic acid, it is dichloroacetic acid, trifluoroacetic acid, propionic acid, butyric acid, malonic acid, oxalic acid, adipic acid, camphorsulfonic acid, methanesulfonic acid, p-toluenesulfonic acid, trans ferulae Acid, salicylic acid, malic acid, succinic acid, p-hydroxybenzoic acid, lactic acid, caffeic acid, chlorogenic acid, sulfanilic acid, 5-sulfosalicylic acid, fumaric acid, gluconic acid, itaconic acid or sorbic acid.
[0038] The specific steps of the preparation method of the 1-methyl-5-bromophylline derivat...
Embodiment 2
[0042] The preparation method of 1-methyl-5-bromophylline benzoate shown in chemical structural formula I-1 is as follows:
[0043] The chemical structural formula I-1 of 1-methyl-5-bromophylline benzoate is
[0044]
[0045] The concrete steps of its preparation method are as follows:
[0046] In the first step, add 5 mL of DMF and 5 mL of formaldehyde solution with a mass percentage concentration of 37% to a 150 mL reactor at a temperature of 0 to 5° C. and magnetic stirring at 600 rpm. % dimethylamine aqueous solution is 33.5mmol of dimethylamine, 2.855mL of glacial acetic acid was added dropwise after 1h, and reacted for 15min, and then 3.20g (15.23mmol) of raw material 1-methyl-5-bromoindole was added, and after 1.5h of reaction, Transfer to room temperature and continue the reaction for 12 hours. After the completion of the reaction was detected by TLC spotting, first add saturated saline, then add a 30% NaOH aqueous solution by mass percentage, adjust the pH of the ...
Embodiment 3
[0049] The preparation method of 1-methyl-5-bromophylline L malate shown in chemical structural formula I-2 is as follows:
[0050] The chemical structural formula I-2 of 1-methyl-5-bromophylline L malate is
[0051]
[0052] The concrete steps of its preparation method are as follows:
[0053] The first step, with embodiment 1;
[0054] In the second step, except that benzoic acid is replaced with L malic acid, the others are the same as in Example 1, and purified by column chromatography to obtain a light yellow oil with a yield of 94%; 1 H NMR (400MHz, DMSO-d 6 )δ7.94(d, J=1.7Hz, 1H, Ar-H), 7.46(s, 1H, Ar-H), 7.44(d, J=8.7Hz, 1H, Ar-H), 7.30(dd, J=1.8,8.7Hz,1H,Ar-H),4.00(s,2H,Ar-CH 2 ),3.86–3.92(m,1H,CH 2 -CH),3.79(s,3H,N-CH 3 ),3.18(s,3H,CO 2 H,OH),2.49–2.56(m,1H,CH-CH 2 ),2.46(s,6H,N-(CH 3 ) 2 ),2.31–2.38(m,1H,CH-CH 2 ); 13 C NMR (100MHz, DMSO-d 6 )δ 177.2, 172.7, 135.8, 132.8, 129.9, 124.4, 121.9, 112.6, 106.5, 66.5, 52.5, 49.1, 43.2, 42.2, 33.2, the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com