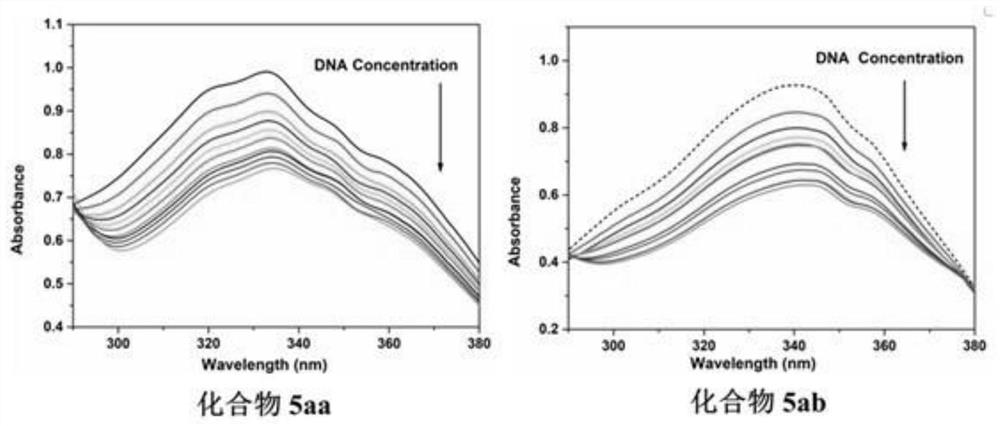
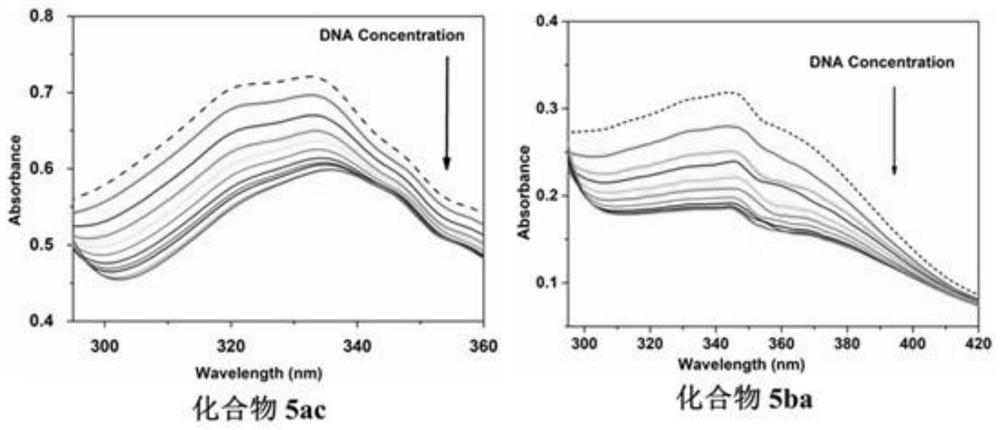
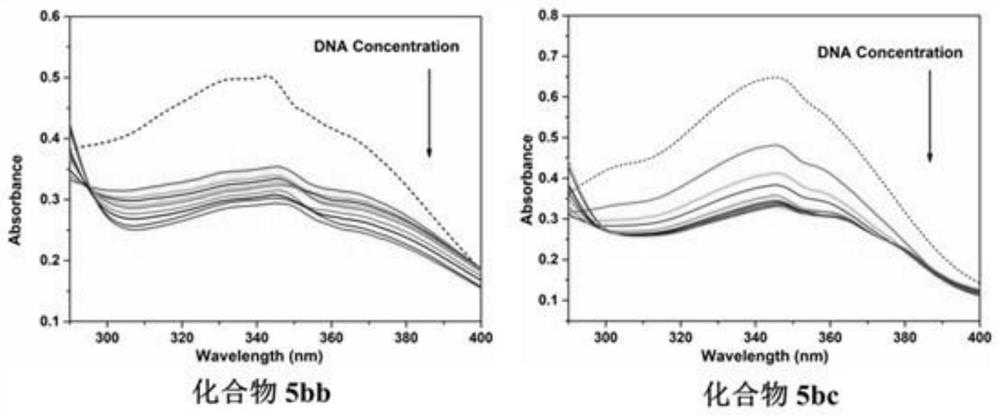
2-p-nitrostyryl-4-substituted aminoquinazoline derivatives and their preparation methods and applications
An aminoquinazoline and nitrostyryl technology, which is applied in the field of 2-p-nitrostyryl-4-substituted aminoquinazoline derivatives and their preparation, can solve the problems of inability to exhibit antitumor activity and the like, and achieve normal Low cytotoxicity, strong antitumor activity, and high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Embodiment 1: preparation compound 2a
[0028] 1) Add anthranilamide in acetic anhydride, the addition of anthranilamide in every milliliter of acetic anhydride is 1.2mmol, fully stir and react at room temperature for 1 hour (TLC monitors the reaction, developer: V 乙酸乙酯 :V 石油醚 =1:2), precipitate solid, obtain reaction mixture; 2) add water in reaction mixture, and suction filtration, get filter residue; Wash filter residue with saturated sodium bicarbonate solution, water and ethyl acetate successively, then wash The good filter residue was dried to obtain the compound 2a, which was a white solid with a yield of 87%.
[0029] m.p.172~174℃; 1 H NMR (400MHz, CD 3 OD)δ: 8.35(d, J=8.3Hz, 1H), 7.72(dd, J=7.9, 1.4Hz, 1H), 7.49~7.43(m, 1H), 7.15~7.11(m, 1H), 2.15( s,3H).(the resonance ofO-H was not observed in CD 3 OD).
[0030] The structural formula of the prepared compound 2a is:
Embodiment 2
[0031] Embodiment 2: preparation compound 2b
[0032] The compound 2-amino-5-chlorobenzamide was used to replace anthranilamide, and the synthesis method was the same as in Example 1 to obtain the compound 2b with a yield of 99%.
[0033] 6-Chloro-2-methylquinazolin-4-ol(2b).White solid, yield 99%, m.p.191~193℃. 1 H NMR (400MHz, CDCl 3 )δ:10.96(s,1H),8.61(d,J=8.9Hz,1H),7.73~7.34(m,1H),2.20(s,3H).(the resonance of O-H was not observed in CDCl 3 ).
[0034] The structural formula of the prepared compound 2b is:
Embodiment 3
[0035] Embodiment 3: preparation compound 3a
[0036] 1) Add compound 2a, p-nitrobenzaldehyde and acetic acid successively in the reaction vessel, the addition of compound 2a in every milliliter of acetic acid is 0.9mmol, the addition of p-nitrobenzaldehyde is 1.2mmol, the reaction vessel is placed in oil In bathing pot, under nitrogen protection, heating and stirring under reflux reaction 12h (TLC monitors reaction, developer: V乙酸乙酯 :V 石油醚 =1:2), cooling; 2) suction filtration, take the filter residue, wash and dry to obtain compound 3a, compound 3a is a light yellow solid, and the yield is 60%.
[0037] m.p.315~317℃. 1 H NMR (400MHz, DMSO-d 6 )δ: 12.44(s, 1H), 8.30(d, J=8.6 Hz, 2H), 8.13(d, J=8.0Hz, 1H), 8.04(d, J=16.2Hz, 1H), 7.93(d, J=8.6Hz, 2H),7.86~7.82(m,1H),7.71(d,J=8.0Hz,1H),7.54~7.50(m,1H),7.20(d,J=16.2Hz,1H).
[0038] The structural formula of the prepared compound 3a is:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com