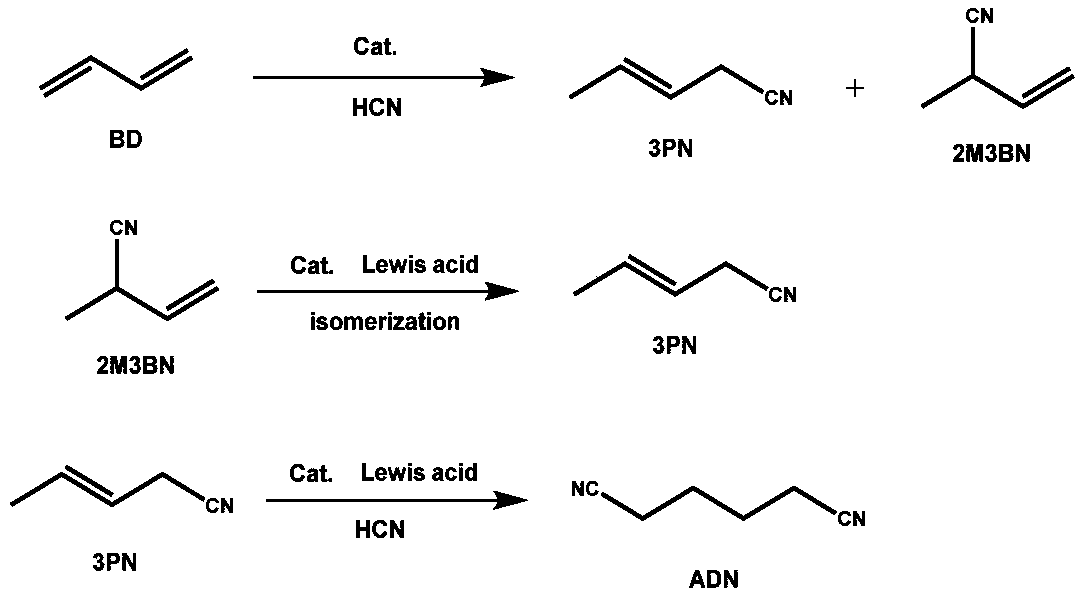
Method for preparing 3-pentenenitrile
A technology for pentene nitrile and butene nitrile is applied in the field of preparing 3-pentene nitrile and can solve the problems such as the decrease of catalytic activity of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] 2-methyl-2-butenenitrile, copper, diisodecyl phosphite, 1,6-bisdiphenylphosphine hexane, magnesium chloride, and xylene at 110:1:20:10:5:30 The molar ratio of the isomerization reactor was added, the reaction pressure was controlled to be 0.5Mpa, and the reaction temperature was 100°C. During the reaction, nitrogen protection was carried out, mechanical stirring was carried out, and the reaction time was 12 hours. The content of each component of the reaction process is detected and analyzed by the conventional gas chromatography analysis method, and the peak area of each material is provided by the gas chromatography in the experimental process as shown in Table 1. In the experiment, n-octane is used as the internal standard. The standard sample of known n-octane and nitriles content is analyzed by chromatography, so that the area relationship between the internal standard and nitriles is as follows:
[0058] Calibration formula for trans-2-methyl-2-butenenitrile: y ...
Embodiment 2
[0076] 2-Methyl-2-butenenitrile, nickel, triphenyl phosphite, 1,2-bisdiphenylphosphineethane, triphenylboron, and benzene in a ratio of 30:1:5:5:1: The molar ratio of 50 was added to the isomerization reactor, the reaction pressure was set at 0.3Mpa, and the reaction temperature was set at 130°C. During the reaction, nitrogen protection was carried out, mechanical stirring was carried out, and the reaction time was 6 hours. After the reaction was completed, unreacted 2-methyl-2-butenenitrile and product 3-pentenenitrile were separated by vacuum distillation . It was detected and analyzed by a conventional gas chromatography analysis method, and the results were: the conversion rate of 2-methyl-2-butenenitrile was 83%, and the selectivity of 3-pentenenitrile was 89.5%.
Embodiment 3
[0078] 2-methyl-2-butenenitrile, cobalt, triisooctyl phosphite, 1,1'-binaphthyl-2,2'-bisdiphenylphosphine, ferric chloride, and dimethylformamide Add the molar ratio of 60:1:10:10:5:30 into the isomerization reactor, control the reaction pressure to 0.2Mpa, and the reaction temperature to 150°C. During the reaction, carry out nitrogen protection, mechanical stirring, and react The time was 18 hours. After the reaction was finished, the unreacted 2-methyl-2-butenenitrile and the product 3-pentenenitrile were separated by distillation under reduced pressure. It was detected and analyzed by a conventional gas chromatography analysis method, and the results were: the conversion rate of 2-methyl-2-butenenitrile was 81%, and the selectivity of 3-pentenenitrile was 89%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com