Method for evaluating demethyltransferase activity of histone lysine
A histone and lysine technology, applied in the field of enzyme activity detection, can solve the problem of inability to monitor the enzyme reaction in real time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] The present invention provides a method for evaluating the activity of histone lysine demethyltransferase containing Jumonji-C domain. In order to make the purpose, technical scheme and effect of the present invention clearer and clearer, the present invention will be further described in detail below. It should be understood that the specific embodiments described here are only used to explain the present invention, not to limit the present invention.
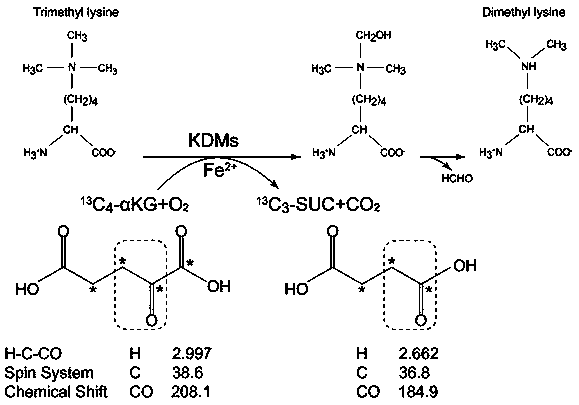
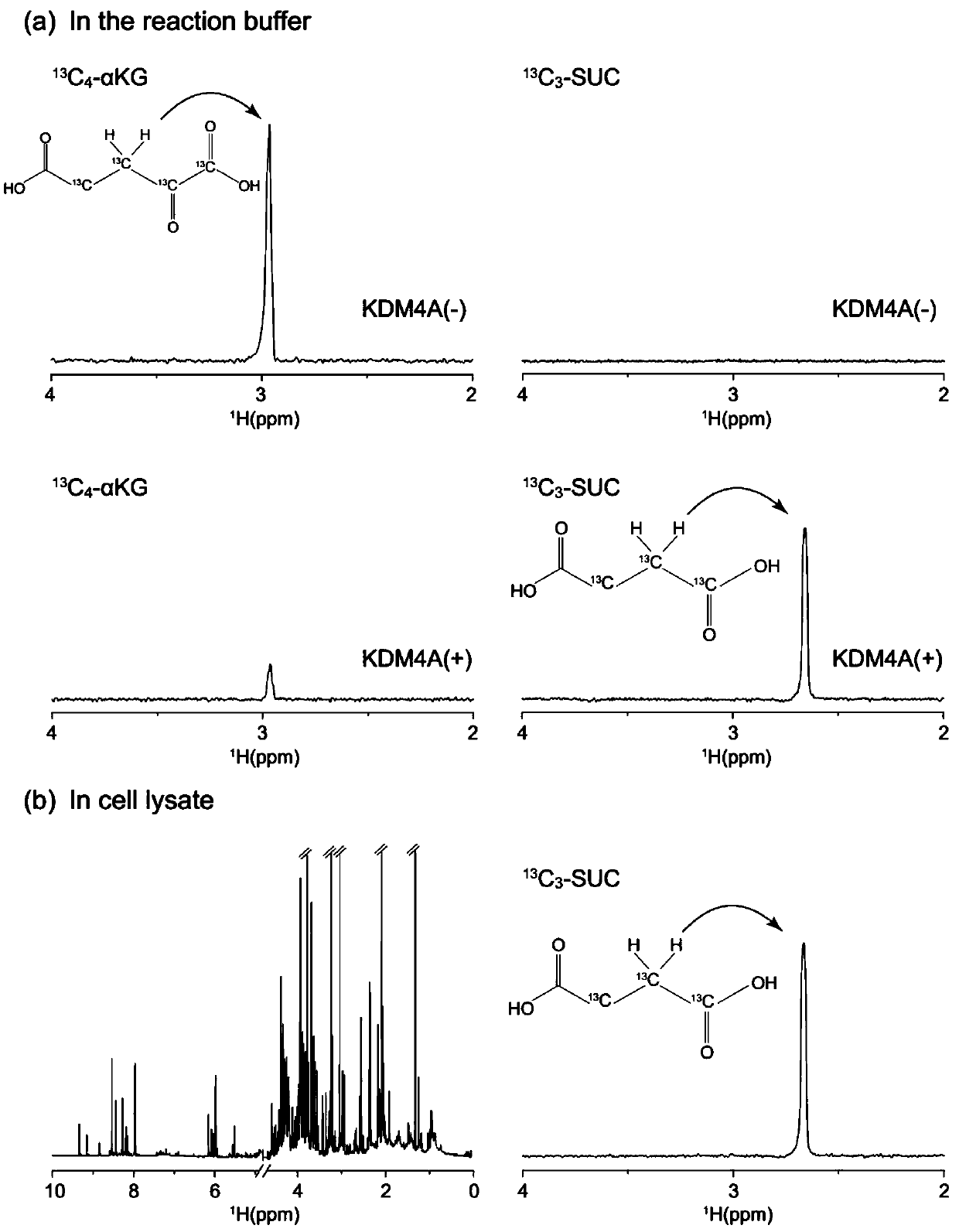
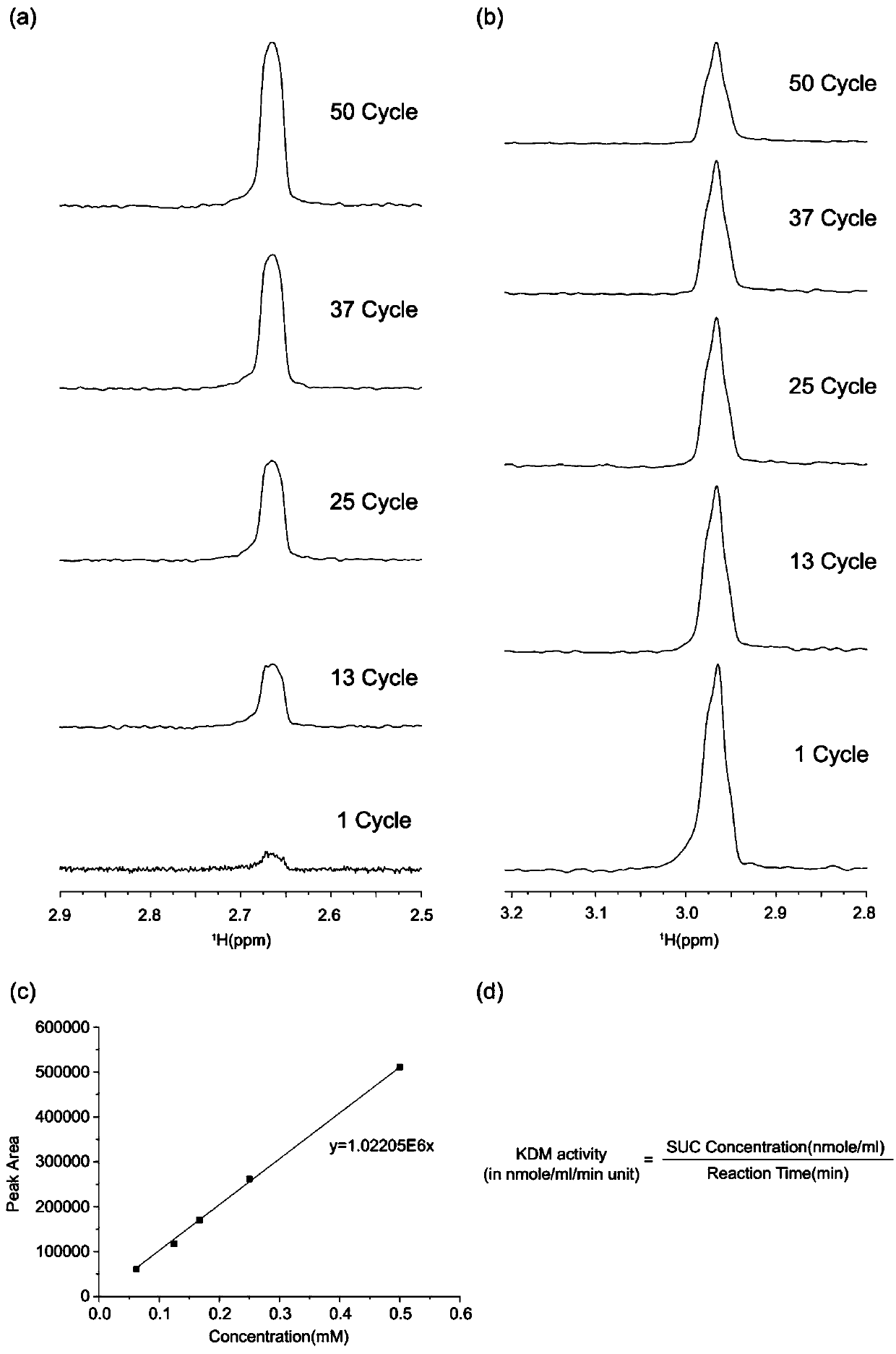
[0020] An embodiment of the present invention provides a method for evaluating the activity of a histone lysine demethyltransferase containing a Jumonji-C domain, wherein carbon-13-labeled α-KG and a nuclear magnetic resonance spectrometer are used in a biological mixture (such as cell lysis Selective real-time monitoring of the changes of substrate α-KG and product succinate of histone lysine demethyltransferase containing Jumonji-C domain in liquid, etc.) to evaluate histone lysine demethylation transfer enzyme activi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com